What Is The Conjugate Acid For Hso4-
listenit
Mar 27, 2025 · 5 min read
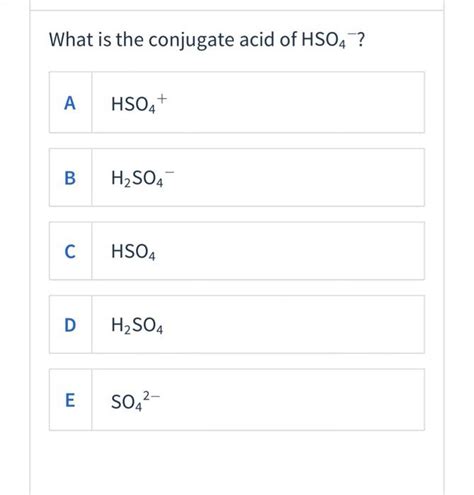
Table of Contents
What is the Conjugate Acid for HSO₄⁻? A Deep Dive into Acid-Base Chemistry
Understanding conjugate acid-base pairs is fundamental to grasping acid-base chemistry. This article delves deep into the concept, focusing specifically on the conjugate acid of the bisulfate ion, HSO₄⁻. We'll explore its formation, properties, and importance in various chemical contexts. We'll also touch upon related concepts to provide a comprehensive understanding.
Understanding Conjugate Acid-Base Pairs
According to Brønsted-Lowry acid-base theory, an acid is a substance that donates a proton (H⁺), while a base is a substance that accepts a proton. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. These pairs are always related by the difference of a single proton.
The equilibrium between an acid and its conjugate base (or a base and its conjugate acid) is a key feature of acid-base reactions. The strength of an acid is directly related to the stability of its conjugate base. A strong acid will have a weak conjugate base, and vice versa.
Identifying the Conjugate Acid of HSO₄⁻
The bisulfate ion, HSO₄⁻, acts as a weak acid in aqueous solutions. This means it partially donates a proton. To find its conjugate acid, we need to add a proton (H⁺) to the HSO₄⁻ ion. This results in:
HSO₄⁻ + H⁺ ⇌ H₂SO₄
Therefore, the conjugate acid of HSO₄⁻ is sulfuric acid (H₂SO₄).
Properties of Sulfuric Acid (H₂SO₄)
Sulfuric acid is a strong diprotic acid, meaning it can donate two protons. Its first dissociation is essentially complete in aqueous solution:
H₂SO₄ → H⁺ + HSO₄⁻
The second dissociation, however, is only partial:
HSO₄⁻ ⇌ H⁺ + SO₄²⁻
This second dissociation is where the HSO₄⁻ ion comes into play, acting as a weak acid. The complete dissociation of sulfuric acid showcases its strength as an acid and highlights the relationship between H₂SO₄ and its conjugate base, HSO₄⁻.
Key Properties of Sulfuric Acid:
- Strong Acid: As mentioned, its first proton dissociation is almost complete.
- Diprotic: It can donate two protons.
- Dehydrating Agent: Sulfuric acid is a powerful dehydrating agent, meaning it can remove water molecules from other substances. This property is used in various industrial processes.
- Oxidizing Agent: Concentrated sulfuric acid acts as an oxidizing agent, particularly at high temperatures.
- Highly Corrosive: It's extremely corrosive and requires careful handling.
The Importance of HSO₄⁻ and H₂SO₄
Both HSO₄⁻ and H₂SO₄ play significant roles in various chemical processes and industrial applications:
Applications of HSO₄⁻:
- Buffer Solutions: HSO₄⁻, along with its conjugate base SO₄²⁻, can be used to create buffer solutions, which resist changes in pH. Buffer solutions are crucial in maintaining stable pH levels in many chemical and biological systems.
- Electrolyte: In some electrochemical applications, HSO₄⁻ acts as an electrolyte, conducting electricity.
- Intermediate in Reactions: HSO₄⁻ frequently participates as an intermediate in various chemical reactions.
Applications of H₂SO₄:
- Industrial Applications: Sulfuric acid is one of the most widely produced chemicals globally. Its immense versatility leads to applications in:
- Fertilizer Production: Used extensively in the production of phosphate fertilizers.
- Petroleum Refining: Employed in alkylation and other refining processes.
- Metal Processing: Used in various metal cleaning and processing procedures.
- Battery Manufacturing: A key component in lead-acid batteries.
- Chemical Synthesis: Utilized as a catalyst and reagent in numerous chemical syntheses.
Understanding Acid Strength and pKa Values
The strength of an acid is often expressed using its pKa value. The pKa is the negative logarithm (base 10) of the acid dissociation constant (Ka). A lower pKa value indicates a stronger acid. For HSO₄⁻, the pKa value is approximately 1.99. This relatively low pKa value signifies that it's a weak but still relatively strong acid compared to many other weak acids. The pKa reflects the equilibrium between HSO₄⁻ and its conjugate base, SO₄²⁻, and the propensity to donate a proton.
The difference in pKa values between sulfuric acid's first and second dissociation steps clearly demonstrates the significant difference in their acid strength. The first dissociation (H₂SO₄ → H⁺ + HSO₄⁻) has a very low pKa (essentially -3), reflecting its complete dissociation. The second dissociation (HSO₄⁻ ⇌ H⁺ + SO₄²⁻) has a pKa of 1.99, indicating partial dissociation.
Comparing HSO₄⁻ to Other Weak Acids
Many other weak acids exist, each with its own unique properties and pKa value. Comparing HSO₄⁻ to other weak acids helps contextualize its strength and reactivity. For example:
- Acetic Acid (CH₃COOH): Acetic acid is a common weak acid with a pKa of 4.76. Its pKa is significantly higher than that of HSO₄⁻, signifying that it is a much weaker acid.
- Phosphoric Acid (H₃PO₄): Phosphoric acid is a triprotic acid with three pKa values (2.15, 7.20, and 12.35). The first dissociation is comparable in strength to the dissociation of HSO₄⁻.
- Hydrofluoric Acid (HF): Hydrofluoric acid is a weak acid with a pKa of 3.17, also weaker than HSO₄⁻.
Further Exploration: Lewis Acid-Base Theory
While the Brønsted-Lowry theory focuses on proton transfer, the Lewis theory provides a broader definition of acids and bases. A Lewis acid is a substance that can accept an electron pair, and a Lewis base is a substance that can donate an electron pair. Sulfuric acid and the bisulfate ion can act as Lewis acids due to the availability of empty orbitals that can accommodate electron pairs. This aspect of their chemistry further highlights their reactivity and potential for participation in diverse reactions.
Conclusion
The conjugate acid of HSO₄⁻ is sulfuric acid (H₂SO₄). Understanding this relationship, along with the properties of both HSO₄⁻ and H₂SO₄, is critical for comprehending various acid-base reactions and their applications. The pKa value provides a quantitative measure of the acid strength, allowing for comparisons with other acids. The broader concept of Lewis acid-base theory provides a more encompassing perspective on the reactivity of these species. By understanding these aspects, one can gain a more comprehensive grasp of the fundamental principles of acid-base chemistry and their significance in diverse chemical contexts. This knowledge is invaluable in various fields, ranging from industrial chemistry and environmental science to biochemistry and medicinal chemistry.
Latest Posts
Latest Posts
-
What Is 1 2 3 4
Mar 30, 2025
-
What Type Of Rock Are Fossils Mostly Found In
Mar 30, 2025
-
During Which Phase Of Meiosis Do Homologous Chromosomes Separate
Mar 30, 2025
-
How Many Chromosomes Do Daughter Cells Have After Mitosis
Mar 30, 2025
-
Is Hydrobromic Acid A Strong Acid
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Acid For Hso4- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
