What Is The Chemical Formula For Barium Phosphate
listenit
Apr 07, 2025 · 5 min read
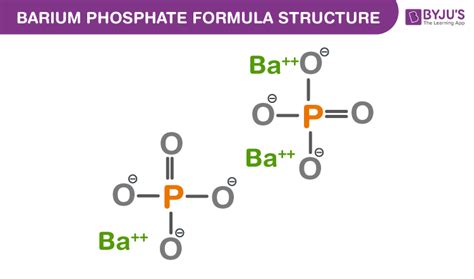
Table of Contents
What is the Chemical Formula for Barium Phosphate?
Barium phosphate is an inorganic compound with several applications in various industries. Understanding its chemical formula is crucial for anyone working with this compound. This comprehensive guide delves into the chemical formula of barium phosphate, exploring its structure, properties, and uses, while also addressing common misconceptions and providing additional context for a deeper understanding.
Understanding Chemical Formulas
Before diving into the specifics of barium phosphate, let's refresh our understanding of chemical formulas. A chemical formula is a concise representation of the atoms present in a molecule or compound. It indicates the types of atoms and their relative ratios within the substance. For example, H₂O represents water, showing that each molecule consists of two hydrogen atoms (H) and one oxygen atom (O).
The chemical formula provides essential information about the compound's composition, allowing chemists and other scientists to understand its properties and predict its behavior.
Determining the Chemical Formula for Barium Phosphate
Barium phosphate is an ionic compound formed from the combination of barium cations (Ba²⁺) and phosphate anions (PO₄³⁻). To determine the correct chemical formula, we need to ensure that the overall charge of the compound is neutral. This principle, known as charge neutrality, requires the positive and negative charges to balance each other.
- Barium (Ba): Barium is an alkaline earth metal with a +2 charge (Ba²⁺).
- Phosphate (PO₄): Phosphate is a polyatomic anion with a -3 charge (PO₄³⁻).
To achieve charge neutrality, we need three barium cations (+6 total charge) to balance the charge of two phosphate anions (-6 total charge). Therefore, the chemical formula for barium phosphate is Ba₃(PO₄)₂.
This formula indicates that each unit of barium phosphate contains three barium atoms and two phosphate groups.
The Structure of Barium Phosphate
The structure of barium phosphate is a complex three-dimensional arrangement of barium cations and phosphate anions. The barium ions are surrounded by phosphate ions, and vice versa, forming a crystalline lattice. The exact arrangement of atoms within this lattice can be complex, often depending on factors such as temperature and pressure. Detailed structural analysis requires sophisticated techniques like X-ray diffraction.
Understanding the crystal structure is essential for comprehending many of the physical and chemical properties of barium phosphate. For instance, the crystal structure dictates its solubility, density, and reactivity.
Properties of Barium Phosphate
Barium phosphate exhibits several key properties, many of which are directly related to its chemical formula and structure. These properties include:
-
Solubility: Barium phosphate is sparingly soluble in water, meaning only a small amount dissolves in water at a given temperature. This low solubility is a crucial factor in its applications.
-
Molar Mass: The molar mass of Ba₃(PO₄)₂ is approximately 601.92 g/mol. This is calculated by summing the atomic masses of its constituent elements (three barium atoms, two phosphorus atoms, and eight oxygen atoms).
-
Appearance: Barium phosphate typically appears as a white crystalline powder.
-
Toxicity: Like many barium compounds, barium phosphate is toxic if ingested. Appropriate safety precautions should always be taken when handling this compound.
-
Reactivity: Barium phosphate is relatively unreactive under normal conditions but may react with strong acids to form soluble barium salts and phosphoric acid.
Applications of Barium Phosphate
The unique properties of barium phosphate make it suitable for various applications across several industries:
-
Phosphor Production: Barium phosphate is a crucial component in the manufacturing of phosphors used in fluorescent lamps and cathode ray tubes (CRTs). When exposed to electron beams or ultraviolet (UV) radiation, these phosphors emit light of various colors.
-
Ceramic Industry: It’s used as a component in certain ceramic materials. Its addition can modify the properties of the final ceramic product.
-
Chemical Catalyst: In some instances, it can act as a catalyst or catalyst support in certain chemical reactions.
-
Pigments: Barium phosphate has found limited use in the creation of specific pigments. However, this is often outweighed by concerns surrounding the toxicity of barium compounds.
-
Medical Applications (Limited): Although barium compounds are known for their radiological applications (barium sulfate in contrast media), barium phosphate's use in medicine is limited due to toxicity concerns.
Common Misconceptions and Clarifications
One common source of confusion revolves around the potential for different forms or hydrates of barium phosphate. While some variations in crystal structure and hydration levels might occur depending on synthesis conditions, Ba₃(PO₄)₂ remains the core chemical formula representing the fundamental compound. Any additional water molecules would be denoted separately in the formula (e.g., Ba₃(PO₄)₂·xH₂O where 'x' represents the number of water molecules per formula unit).
It's essential to distinguish barium phosphate from other barium compounds, such as barium sulfate (BaSO₄), which has very different properties and applications (often used as a radiocontrast agent). The difference in the anion drastically alters the solubility and toxicity.
Conclusion: A Deeper Look at Ba₃(PO₄)₂
The chemical formula for barium phosphate, Ba₃(PO₄)₂, is a concise yet powerful representation of this inorganic compound. Understanding this formula is essential for anyone working with or studying this compound. This knowledge allows for a better understanding of its properties, applications, and potential hazards. Remember always to handle barium phosphate with care and follow appropriate safety protocols to prevent accidental exposure. Further research into the crystal structure and its influence on reactivity and specific application contexts will offer an even deeper understanding of this fascinating inorganic compound. The information presented here provides a solid foundation for further exploration and learning about barium phosphate and its role in various industries.
Latest Posts
Latest Posts
-
What Is X 1 X 1
Apr 08, 2025
-
What Is The Gcf Of 8 12
Apr 08, 2025
-
Dna Is Called The Blueprint Of Life Because
Apr 08, 2025
-
Difference Between Molar Mass And Atomic Mass
Apr 08, 2025
-
What Is The Greatest Common Factor Of 64 And 32
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about What Is The Chemical Formula For Barium Phosphate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.