What Is The Charge Of Ni
listenit
Mar 21, 2025 · 5 min read
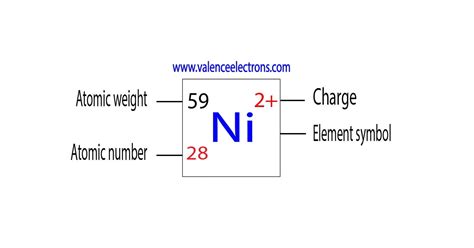
Table of Contents
What is the Charge of Ni? Understanding Nickel's Oxidation States and Chemistry
Nickel (Ni), a silvery-white metal, is a fascinating element with a rich history and diverse applications. While often perceived as a single entity, understanding its chemical behavior requires delving into its various oxidation states. This article will comprehensively explore the charge of Ni, delving into its common oxidation states, their stability, and the factors influencing them. We'll also touch upon the implications of these varying charges in different chemical contexts and its wider impact.
The Versatile Nature of Nickel's Charge
Unlike some elements that exhibit a limited range of oxidation states, nickel demonstrates a remarkable versatility, readily existing in several oxidation states. However, two stand out as the most prevalent: +2 and 0.
Nickel(II) (+2): The Dominant Oxidation State
The +2 oxidation state, represented as Ni²⁺, is by far the most common and stable oxidation state for nickel. This is due to the electronic configuration of the nickel atom and its tendency to lose two electrons to achieve a stable, fully filled 3d orbital. This stability is a key factor in its widespread presence in various compounds and its diverse applications.
-
Examples of Ni(II) Compounds: Numerous compounds showcase nickel in its +2 oxidation state. These include nickel(II) oxide (NiO), a green-black powder used in ceramics and batteries; nickel(II) chloride (NiCl₂), a yellow-green crystalline solid employed in electroplating and as a catalyst; and nickel(II) sulfate (NiSO₄), a green crystalline solid used in nickel plating solutions. The diverse colors exhibited by these compounds are often attributed to the influence of the ligands surrounding the nickel ion.
-
Stability and Reactivity: The stability of Ni²⁺ is evident in its relatively inert nature in many aqueous solutions. However, its reactivity varies depending on the ligand environment. Certain ligands can influence the reactivity and even induce changes in the coordination geometry surrounding the nickel ion. Understanding these interactions is crucial in areas such as catalysis and coordination chemistry.
Nickel(0): Elemental Nickel and its Role
Nickel(0) refers to elemental nickel, in which the nickel atom has a zero oxidation state (Ni⁰). In this form, nickel displays its characteristic metallic properties: malleability, ductility, and excellent conductivity. It is in this state that nickel finds extensive use in various industrial applications.
- Applications of Nickel(0): Pure nickel finds applications in various alloys, such as stainless steel and nickel-based superalloys, prized for their corrosion resistance and high-temperature strength. It's a critical component in many industrial processes, including catalysis (e.g., Raney nickel), and is also used in batteries and coinage.
Other Oxidation States: Less Common but Significant
While less prevalent than +2 and 0, nickel can exist in other oxidation states, albeit often under specific conditions. These include:
-
Nickel(I) (+1): Ni⁺ is relatively rare and less stable than Ni²⁺. It is typically observed in complexes with strong-field ligands that stabilize the +1 state.
-
Nickel(III) (+3): Ni³⁺ is even less common than Ni⁺ and is usually found in complexes with strong oxidizing ligands that help stabilize this higher oxidation state. Its existence is often fleeting and requires specific conditions.
-
Nickel(IV) (+4): This is the highest oxidation state observed for nickel and is extremely rare. It's mostly found in complexes with very strong oxidizing ligands, often under rigorously controlled conditions.
Factors Influencing Nickel's Oxidation State
Several factors contribute to the prevalence of certain nickel oxidation states over others:
-
Ligand Field Effects: The ligands surrounding the nickel ion strongly influence its oxidation state. Strong-field ligands can stabilize higher oxidation states like +3 or +4, while weak-field ligands favor the +2 state. This is directly related to crystal field theory and ligand field stabilization energy.
-
pH: The acidity or basicity of the solution can affect the stability of different nickel oxidation states. Changes in pH can lead to the formation or precipitation of nickel compounds in different oxidation states.
-
Redox Potential: The redox potential of the system plays a crucial role in determining which oxidation state is favored. A more oxidizing environment can favor higher oxidation states, while a reducing environment stabilizes lower oxidation states.
-
Temperature: Temperature can also influence the equilibrium between different oxidation states. Higher temperatures may favor higher oxidation states in some cases.
The Significance of Understanding Nickel's Charge
Understanding the various oxidation states of nickel and the factors that influence them is critical in several scientific and technological fields:
-
Catalysis: Nickel's ability to exist in different oxidation states is crucial for its catalytic activity. Many nickel-based catalysts rely on changes in nickel's oxidation state during the catalytic cycle.
-
Electrochemistry: Nickel's electrochemical behavior is directly related to its ability to undergo redox reactions, involving changes in its oxidation state. This is fundamental to nickel-cadmium and nickel-metal hydride batteries.
-
Materials Science: The oxidation state of nickel significantly impacts the properties of nickel-containing materials. The choice of oxidation state can influence the material's stability, conductivity, and magnetic properties.
-
Biological Systems: Nickel plays a role in certain biological systems, often involving the +2 oxidation state. Understanding its chemistry in these systems is important for comprehending biological processes and potential applications in biomedicine.
-
Environmental Chemistry: The understanding of nickel’s oxidation states is relevant in environmental contexts. For example, the mobility and bioavailability of nickel in soil and water are influenced by its oxidation state and the chemical species it forms. Different oxidation states exhibit varying toxicity levels, influencing environmental risk assessment.
Conclusion: A Deeper Dive into Nickel's Chemistry
The charge of nickel, far from being a simple +2, reflects a diverse and dynamic chemical landscape. Its ability to exist in multiple oxidation states, predominantly +2 and 0, highlights its versatility and explains its broad range of applications. The factors influencing these oxidation states – ligand field effects, pH, redox potential, and temperature – provide a rich tapestry of chemical complexities. A thorough understanding of these intricacies is essential not only for researchers in materials science, chemistry, and environmental science, but also for professionals working in various industries utilizing nickel and its compounds. Further research into the less common oxidation states of nickel continues to reveal new insights and potential applications, solidifying nickel's position as a pivotal element in modern science and technology. This detailed examination emphasizes the importance of considering the full range of nickel's oxidation states to fully appreciate its diverse roles and potential.
Latest Posts
Latest Posts
-
When To Use The Ratio Test
Mar 21, 2025
-
What Are The Common Factors Of 24 And 40
Mar 21, 2025
-
How Do You Measure Density Of A Solid
Mar 21, 2025
-
What Is The Most Reactive Element
Mar 21, 2025
-
What Is The Electron Configuration For Ne
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Ni . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
