What Is The Charge Of An Ionic Compound
listenit
Apr 07, 2025 · 6 min read
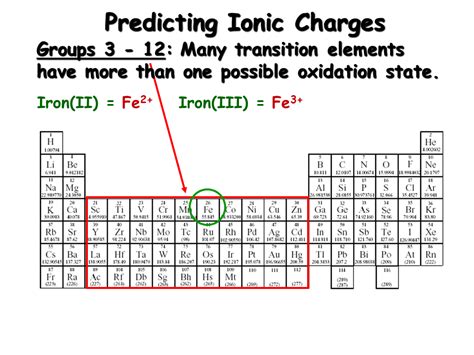
Table of Contents
What is the Charge of an Ionic Compound? Understanding Ionic Bonding and Net Charge
Ionic compounds are fundamental to chemistry, forming the basis of many materials and biological processes. Understanding their charge is crucial for comprehending their properties and behavior. This comprehensive guide delves into the intricacies of ionic bonding, explaining how the charges of individual ions combine to determine the overall charge of an ionic compound.
The Essence of Ionic Bonding: A Dance of Opposite Charges
Ionic bonding arises from the electrostatic attraction between oppositely charged ions. This occurs when one atom, typically a metal, readily donates electrons, becoming a positively charged cation, while another atom, usually a non-metal, readily accepts those electrons, becoming a negatively charged anion. This electron transfer aims to achieve a stable electron configuration, often resembling that of a noble gas.
Understanding Cations and Anions: The Building Blocks of Ionic Compounds
-
Cations: Positively charged ions formed when an atom loses electrons. Metals, with their relatively low electronegativity, tend to lose electrons easily, forming cations. The charge of a cation is determined by the number of electrons lost. For example, sodium (Na) loses one electron to become Na⁺, while magnesium (Mg) loses two electrons to become Mg²⁺.
-
Anions: Negatively charged ions formed when an atom gains electrons. Non-metals, with their higher electronegativity, tend to gain electrons to fill their outermost electron shell. The charge of an anion is determined by the number of electrons gained. For example, chlorine (Cl) gains one electron to become Cl⁻, while oxygen (O) gains two electrons to become O²⁻.
The Electrostatic Force: The Glue that Holds Ionic Compounds Together
The fundamental force driving the formation and stability of ionic compounds is the strong electrostatic attraction between the positively charged cations and the negatively charged anions. This attraction overcomes the repulsive forces between like charges (cation-cation and anion-anion), resulting in a stable, three-dimensional crystalline structure.
Determining the Overall Charge of an Ionic Compound: The Principle of Electroneutrality
The defining characteristic of an ionic compound is its overall neutral charge. This means that the total positive charge from the cations precisely balances the total negative charge from the anions. This principle of electroneutrality is paramount in understanding the formula and properties of ionic compounds.
Applying Electroneutrality: A Step-by-Step Approach
Let's illustrate this with examples:
1. Sodium Chloride (NaCl):
- Sodium (Na) forms a +1 cation (Na⁺).
- Chlorine (Cl) forms a -1 anion (Cl⁻).
To achieve electroneutrality, one Na⁺ ion is required to balance one Cl⁻ ion. Therefore, the formula for sodium chloride is NaCl, reflecting a 1:1 ratio of cations to anions. The overall charge is 0 (+1 + (-1) = 0).
2. Magnesium Oxide (MgO):
- Magnesium (Mg) forms a +2 cation (Mg²⁺).
- Oxygen (O) forms a -2 anion (O²⁻).
One Mg²⁺ ion balances one O²⁻ ion because the charges are numerically equal and opposite. The formula is MgO, and the overall charge is 0 (+2 + (-2) = 0).
3. Aluminum Oxide (Al₂O₃):
- Aluminum (Al) forms a +3 cation (Al³⁺).
- Oxygen (O) forms a -2 anion (O²⁻).
This requires some balancing. To neutralize the +3 charge of aluminum, we need three -2 charges from oxygen. This necessitates two Al³⁺ ions (total charge +6) and three O²⁻ ions (total charge -6). Therefore, the formula is Al₂O₃. The overall charge is 0 (2(+3) + 3(-2) = 0).
4. Calcium Chloride (CaCl₂):
- Calcium (Ca) forms a +2 cation (Ca²⁺).
- Chlorine (Cl) forms a -1 anion (Cl⁻).
One Ca²⁺ ion needs two Cl⁻ ions to balance its +2 charge. Hence, the formula is CaCl₂. The overall charge is 0 (+2 + 2(-1) = 0).
Beyond Simple Compounds: Polyatomic Ions and Complex Structures
While the examples above showcase simple ionic compounds, many involve polyatomic ions. These ions are groups of atoms covalently bonded together, carrying an overall charge.
Polyatomic Ions: Adding Complexity to the Charge Balance
Common polyatomic ions include:
- Nitrate (NO₃⁻): A -1 charged ion.
- Sulfate (SO₄²⁻): A -2 charged ion.
- Phosphate (PO₄³⁻): A -3 charged ion.
- Ammonium (NH₄⁺): A +1 charged ion.
When dealing with polyatomic ions, the same principle of electroneutrality applies. The total positive charge from the cations must equal the total negative charge from the anions, including polyatomic anions.
Example: Ammonium Nitrate (NH₄NO₃):
- Ammonium (NH₄⁺) is a +1 cation.
- Nitrate (NO₃⁻) is a -1 anion.
One NH₄⁺ ion balances one NO₃⁻ ion, resulting in the formula NH₄NO₃ and an overall charge of 0.
Example: Calcium Phosphate [Ca₃(PO₄)₂]:
- Calcium (Ca²⁺) is a +2 cation.
- Phosphate (PO₄³⁻) is a -3 anion.
To balance the charges, we need three Ca²⁺ ions (total charge +6) and two PO₄³⁻ ions (total charge -6). This yields the formula Ca₃(PO₄)₂. The overall charge is 0 (3(+2) + 2(-3) = 0).
Predicting the Charge of Ions: Using the Periodic Table
The periodic table provides a valuable tool for predicting the charge of common ions. The group number (vertical column) often indicates the number of valence electrons, which are the electrons involved in bonding.
- Group 1 (Alkali Metals): Tend to lose one electron, forming +1 cations (e.g., Li⁺, Na⁺, K⁺).
- Group 2 (Alkaline Earth Metals): Tend to lose two electrons, forming +2 cations (e.g., Mg²⁺, Ca²⁺, Ba²⁺).
- Group 17 (Halogens): Tend to gain one electron, forming -1 anions (e.g., Cl⁻, Br⁻, I⁻).
- Group 16 (Chalcogens): Tend to gain two electrons, forming -2 anions (e.g., O²⁻, S²⁻, Se²⁻).
Transition metals and other elements can exhibit variable oxidation states (charges), making their ion charge prediction more complex. These often require knowledge of specific reactions and oxidation-reduction processes.
Implications of Ionic Charge: Properties and Applications
The charge of an ionic compound significantly influences its physical and chemical properties. These properties are critical in numerous applications:
-
Solubility: Ionic compounds often dissolve in polar solvents like water, due to the interaction between the charged ions and the polar water molecules. Solubility is influenced by the strength of the ionic bonds and the size and charge of the ions.
-
Melting and Boiling Points: Ionic compounds typically have high melting and boiling points due to the strong electrostatic forces holding the ions together. Overcoming these forces requires significant energy input.
-
Electrical Conductivity: In their molten state or dissolved in solution, ionic compounds conduct electricity because the freely moving ions can carry an electric current. Solid ionic compounds generally do not conduct electricity.
-
Crystal Structure: The arrangement of ions in an ionic compound's crystal lattice is determined by the size and charge of the ions, leading to different crystal structures with varying properties.
Conclusion: Mastering the Charge of Ionic Compounds
Understanding the charge of an ionic compound is a cornerstone of chemistry. The principle of electroneutrality, guided by the charges of individual ions (both monoatomic and polyatomic), dictates the formula and properties of these substances. By mastering the concepts presented here, you can confidently predict ionic compound charges, understand their behavior, and appreciate their crucial role in various scientific fields and technological applications. The periodic table serves as a valuable tool in this process, while recognizing the exceptions and complexities, especially with transition metals and polyatomic ions, enhances a more complete understanding.
Latest Posts
Latest Posts
-
How Many Electrons Are In Mg 2
Apr 07, 2025
-
Whats 3 5 As A Decimal
Apr 07, 2025
-
In Glycolysis What Is Oxidized And What Is Reduced
Apr 07, 2025
-
A Word That Describes Or Modifies A Noun
Apr 07, 2025
-
What Stores Food And Water In A Cell
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of An Ionic Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.