What Is The Charge For Iron
listenit
Mar 26, 2025 · 5 min read
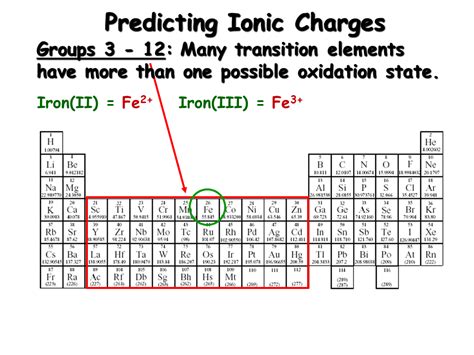
Table of Contents
What is the Charge for Iron? Understanding Oxidation States and Their Significance
Iron, a ubiquitous element crucial to life and industry, doesn't possess a single, fixed charge. Instead, its charge, more accurately referred to as its oxidation state, varies depending on its chemical environment and bonding partners. Understanding iron's variable oxidation states is fundamental to comprehending its diverse roles in biological systems and its numerous applications in materials science and engineering. This article will delve into the intricacies of iron's charge, exploring its common oxidation states, the factors influencing them, and their implications across various fields.
The Nature of Oxidation States
Before we explore iron's specific oxidation states, let's clarify the concept. Oxidation state, or oxidation number, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a useful bookkeeping tool in chemistry, helping us predict reactivity, balance chemical equations, and understand the electronic structure of compounds. It's important to remember that oxidation states are not necessarily the true charges on atoms within a molecule, especially in covalent compounds where electrons are shared.
Key points about oxidation states:
- They can be positive, negative, or zero. A positive oxidation state indicates a loss of electrons, while a negative oxidation state signifies a gain of electrons. An oxidation state of zero implies a neutral atom.
- They are assigned according to a set of rules. These rules are based on electronegativity and the typical bonding behavior of elements.
- They are useful for predicting reactivity and balancing redox reactions. Redox reactions involve the transfer of electrons, and oxidation states help track these electron transfers.
Common Oxidation States of Iron
Iron (Fe), with its atomic number 26 and electronic configuration [Ar] 3d⁶ 4s², exhibits a remarkable versatility in its oxidation states. While it can theoretically exhibit oxidation states ranging from -2 to +6, the most common and significant are +2 and +3.
Iron(II) (Ferrous): +2 Oxidation State
In the +2 oxidation state, iron loses two electrons, typically from its 4s and one 3d orbital. Iron(II) compounds often exhibit a pale green color in aqueous solutions. This oxidation state is relatively less stable than the +3 state, easily undergoing oxidation to Fe(III) in the presence of oxidizing agents.
Examples of Iron(II) Compounds:
- Iron(II) sulfate (FeSO₄): Used as a dietary supplement to treat iron deficiency anemia.
- Iron(II) chloride (FeCl₂): Used as a reducing agent in some chemical processes.
- Iron(II) oxide (FeO): A component of some iron ores.
Iron(III) (Ferric): +3 Oxidation State
Iron(III) is formed when iron loses three electrons, typically from its 4s and two 3d orbitals. Iron(III) compounds often have a rust-brown or yellowish-brown color. This oxidation state is generally more stable than the +2 state, although it can be reduced to Fe(II) under specific conditions.
Examples of Iron(III) Compounds:
- Iron(III) oxide (Fe₂O₃): The main component of rust and a key ingredient in various pigments.
- Iron(III) chloride (FeCl₃): Used as a catalyst in organic chemistry and in water treatment.
- Hematite (α-Fe₂O₃): A major iron ore mineral.
Other, Less Common Oxidation States
While +2 and +3 are the dominant oxidation states, iron can exhibit other, less common oxidation states under specific conditions. These include:
- Iron(0): Found in elemental iron (pure iron metal).
- Iron(IV): Relatively rare, observed in some highly oxidizing environments and certain specialized compounds.
- Iron(VI): Even rarer than Fe(IV), mostly found in ferrates, which are strong oxidizing agents.
Factors Affecting Iron's Oxidation State
Several factors influence which oxidation state iron adopts in a given situation:
- The nature of the ligands: Ligands are the atoms, ions, or molecules surrounding the central iron ion in a complex. Different ligands have different abilities to stabilize different oxidation states. For example, strong-field ligands tend to stabilize higher oxidation states, while weak-field ligands favor lower oxidation states.
- pH: The acidity or basicity of the solution plays a crucial role. Lower pH (more acidic) conditions generally favor higher oxidation states.
- The presence of oxidizing or reducing agents: Oxidizing agents promote higher oxidation states, while reducing agents favor lower oxidation states.
- Temperature and pressure: These factors can also influence the stability of different oxidation states, particularly at extreme conditions.
Significance of Iron's Variable Oxidation States
The ability of iron to exist in multiple oxidation states is critical for its diverse roles:
Biological Roles
Iron plays a vital role in numerous biological processes, primarily through its involvement in electron transfer reactions. The ability to switch between +2 and +3 oxidation states is essential for:
- Oxygen transport: Hemoglobin and myoglobin, which transport oxygen throughout the body, utilize iron in their heme groups to bind and release oxygen. This involves changes in iron's oxidation state.
- Electron transport chain: Iron-sulfur clusters are critical components of the electron transport chain, involved in cellular respiration. These clusters undergo redox reactions, changing iron's oxidation state during electron transfer.
- Enzyme catalysis: Many enzymes require iron as a cofactor, utilizing its variable oxidation states to facilitate catalytic reactions. Cytochrome P450 enzymes, involved in metabolism, are examples.
Industrial Applications
Iron's variable oxidation states are crucial in its industrial applications:
- Steelmaking: The production of steel involves carefully controlling the oxidation state of iron to achieve desired properties like strength and durability.
- Catalysis: Iron compounds, particularly iron oxides, are used as catalysts in various industrial processes, including ammonia production (Haber-Bosch process).
- Pigments: Iron oxides are used extensively as pigments in paints, cosmetics, and other applications, with the color depending on the oxidation state of iron.
- Batteries: Iron is used in various battery technologies, with its oxidation state changing during the charge-discharge cycles.
Conclusion
The charge, or more precisely the oxidation state, of iron is not a fixed value but rather a variable property that depends on its chemical environment. The ability of iron to exist in multiple oxidation states, predominantly +2 and +3, is fundamental to its crucial roles in biological systems and its wide-ranging industrial applications. Understanding the factors influencing iron's oxidation state is key to controlling its properties and harnessing its versatility in various technological advancements. From the transport of oxygen in our blood to the production of steel, iron's variable charge underpins countless essential processes and materials. Further research continues to unveil the complexities and significance of iron's fluctuating oxidation states, promising advancements across multiple scientific and technological domains.
Latest Posts
Latest Posts
-
What Number Is 45 Of 90
Mar 29, 2025
-
What Is 2 5 As A Decimal
Mar 29, 2025
-
18 As A Percentage Of 60
Mar 29, 2025
-
Is Hydrogen Gas At Room Temperature
Mar 29, 2025
-
Solving A System By The Addition Method
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge For Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
