What Is The Center Of The Atom Called
listenit
Mar 17, 2025 · 6 min read
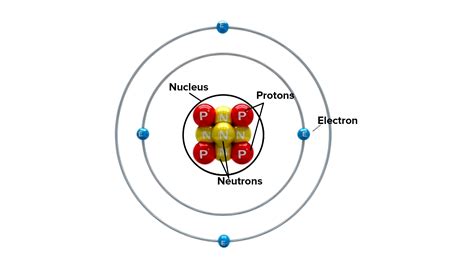
Table of Contents
What is the Center of the Atom Called? Delving into the Nucleus
The atom, the fundamental building block of matter, is a fascinating world of subatomic particles and forces. But what lies at the heart of this tiny universe? The answer, simply put, is the nucleus. This article will delve deep into the nucleus of the atom, exploring its composition, properties, and the crucial role it plays in determining the characteristics of elements. We’ll unravel the mysteries of protons, neutrons, and the strong nuclear force, explaining how these components contribute to the atom's stability and its place in the periodic table.
Understanding the Atom's Structure: A Brief Overview
Before focusing on the nucleus, it's beneficial to briefly recap the overall structure of an atom. Atoms are composed of three fundamental subatomic particles:
- Protons: Positively charged particles residing within the nucleus.
- Neutrons: Neutral particles (no charge) also found in the nucleus.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels.
The vast majority of an atom's volume is empty space, with the electrons occupying this space in a probabilistic cloud around the dense, central nucleus. This model, often visualized as a miniature solar system, provides a basic understanding of atomic structure, though the reality is more nuanced and governed by quantum mechanics.
The Nucleus: The Atom's Dense Core
The nucleus, the central core of the atom, is incredibly small but incredibly dense. It contains virtually all the atom's mass, concentrated in a space a fraction of the atom's overall size. This density is what makes the nucleus so significant in determining an atom's properties. The diameter of the nucleus is only about 1/100,000th of the diameter of the entire atom. Imagine a football stadium – the nucleus would be like a pea in the center, with the electrons scattered throughout the stadium!
The nucleus's density is a result of the strong force, a fundamental force of nature that holds the protons and neutrons together. Without this strong force, the repulsive electromagnetic force between the positively charged protons would cause the nucleus to instantly fly apart.
Protons: The Defining Characteristic of an Element
Protons, with their positive charge, are fundamental to understanding the identity of an element. The number of protons in an atom's nucleus, known as the atomic number, uniquely defines the element. For instance, an atom with one proton is hydrogen, an atom with two protons is helium, and so on. This number is constant for a given element and never changes under normal chemical processes. Changes in the number of protons lead to a transformation into a different element entirely.
Protons contribute significantly to an atom's mass, each possessing approximately one atomic mass unit (amu). Their positive charge is crucial for the atom's overall electrical neutrality, counteracting the negative charge of the electrons.
Neutrons: Contributing to Nuclear Stability
Neutrons, unlike protons, carry no electrical charge. Their presence in the nucleus is essential for nuclear stability, particularly in heavier atoms. The strong nuclear force binds neutrons and protons together, but the electromagnetic repulsion between protons increases with their number. Neutrons help to dilute this repulsive force, preventing the nucleus from falling apart.
The number of neutrons in an atom's nucleus can vary, even within the same element. Atoms with the same number of protons but differing numbers of neutrons are called isotopes. Some isotopes are stable, while others are radioactive, meaning they undergo decay, transforming into different elements over time. This decay process often involves the emission of particles or energy.
The Strong Nuclear Force: The Glue Holding the Nucleus Together
The strong nuclear force is a fundamental force that acts within the nucleus, overcoming the electromagnetic repulsion between protons and binding protons and neutrons together. This force is much stronger than the electromagnetic force at very short distances, but its influence decreases rapidly with increasing distance. This short-range nature is why the nucleus is so compact. Understanding the strong nuclear force is critical for comprehending nuclear reactions, such as nuclear fusion and fission.
Isotopes and Their Significance
As mentioned earlier, isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number affects the atom's mass and stability. Some isotopes are stable and exist naturally in abundance, while others are radioactive and decay over time. Radioactive isotopes have various applications in medicine, research, and industrial processes.
For example, Carbon-12 (⁶C) is a stable isotope with 6 protons and 6 neutrons, while Carbon-14 (¹⁴C) is a radioactive isotope with 6 protons and 8 neutrons. The radioactive decay of ¹⁴C is used in radiocarbon dating to determine the age of organic materials.
Nuclear Reactions: Fusion and Fission
The nucleus is the site of nuclear reactions, which involve changes in the composition of the nucleus. Two main types of nuclear reactions are:
-
Nuclear Fusion: This process involves the combination of two lighter atomic nuclei to form a heavier nucleus. Fusion releases a tremendous amount of energy, as seen in the sun and other stars. This process requires extremely high temperatures and pressures to overcome the electrostatic repulsion between the positively charged nuclei.
-
Nuclear Fission: This involves the splitting of a heavy atomic nucleus into two or more lighter nuclei. Fission also releases a substantial amount of energy, which is harnessed in nuclear power plants. However, fission reactions can also produce radioactive waste, posing environmental challenges.
The Nucleus and the Periodic Table
The periodic table organizes elements based on their atomic number, which directly reflects the number of protons in the nucleus. The arrangement of elements in the periodic table is a testament to the fundamental role of the nucleus in determining the chemical properties of elements. Elements in the same column (group) share similar chemical properties due to similarities in their electron configurations, which are, in turn, influenced by the nuclear charge.
Exploring Further: Beyond the Basics
The study of the nucleus extends far beyond the basics outlined here. Researchers continue to investigate the intricate details of nuclear structure, nuclear forces, and nuclear reactions. Areas of ongoing research include:
- Nuclear Spectroscopy: The study of the energy levels within the nucleus.
- Nuclear Astrophysics: The study of nuclear processes in stars and other celestial objects.
- Nuclear Medicine: The use of radioactive isotopes for diagnosis and treatment of diseases.
- Nuclear Engineering: The application of nuclear principles in the design and operation of nuclear reactors and other technologies.
Conclusion: The Nucleus – A Tiny World of Immense Importance
The nucleus, though small, is the heart of the atom and the key to understanding the properties of matter. Its composition, structure, and the forces governing it determine the characteristics of elements, drive nuclear reactions, and have profound implications for various scientific fields. From the energy powering the sun to the tools used in medical diagnostics, the nucleus plays a crucial role in shaping our world. Continued research into the nucleus will undoubtedly reveal even more about its complexities and its far-reaching influence. The journey into the heart of the atom remains a captivating and ever-evolving area of scientific inquiry.
Latest Posts
Latest Posts
-
10 Is 20 Percent Of What Number
Mar 17, 2025
-
How To Graph X 2 2
Mar 17, 2025
-
What Is 70 Inches In Cm
Mar 17, 2025
-
How Many Degrees Fahrenheit Is One Degree Celsius
Mar 17, 2025
-
1 8 As A Percent And Decimal
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Center Of The Atom Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
