What Is An Atom With A Positive Charge Called
listenit
Mar 19, 2025 · 6 min read
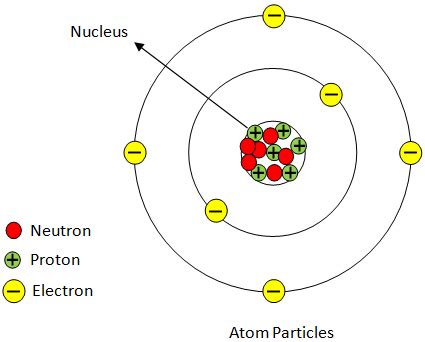
Table of Contents
What is an Atom with a Positive Charge Called? Understanding Ions and Their Significance
An atom with a positive charge is called a cation. This seemingly simple answer opens the door to a fascinating world of chemistry, physics, and material science. Understanding cations is crucial for grasping the fundamental principles governing chemical reactions, the behavior of materials, and numerous technological applications. This article delves deep into the concept of cations, exploring their formation, properties, and widespread importance across various scientific fields.
The Basics of Atomic Structure and Charge
Before we dive into cations, let's briefly review the fundamental building blocks of matter. Atoms, the smallest units of an element that retain its chemical properties, are composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element (e.g., an atom with one proton is hydrogen, two protons is helium, etc.).
- Neutrons: Neutrally charged particles also found in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The electrical charge of an atom is determined by the balance between its protons and electrons. A neutral atom possesses an equal number of protons and electrons, resulting in a net charge of zero. However, atoms can gain or lose electrons, leading to the formation of ions.
Cations: The Positively Charged Ions
A cation is formed when a neutral atom loses one or more electrons. This loss of negatively charged electrons leaves the atom with more protons than electrons, resulting in a net positive charge. The magnitude of the positive charge is equal to the number of electrons lost. For instance:
- A sodium atom (Na) with 11 protons and 11 electrons loses one electron to become a sodium cation (Na⁺), with a +1 charge.
- A magnesium atom (Mg) with 12 protons and 12 electrons can lose two electrons to become a magnesium cation (Mg²⁺), with a +2 charge.
- An aluminum atom (Al) with 13 protons and 13 electrons can lose three electrons to become an aluminum cation (Al³⁺), with a +3 charge.
The tendency of an atom to lose electrons and form cations is primarily determined by its electronic configuration and its position in the periodic table. Elements located on the left side of the periodic table (alkali metals and alkaline earth metals) readily lose electrons to achieve a stable electron configuration (usually a full outermost electron shell), thus forming cations.
Formation of Cations: The Driving Force
The formation of cations is an energetically favorable process, driven by the atom's desire to achieve a more stable electronic configuration. Atoms with incomplete outer electron shells are inherently less stable than those with full outer shells. By losing electrons, these atoms achieve a lower energy state, which is a more stable and favorable condition. This principle is often referred to as the octet rule, although it is not universally applicable.
The energy required to remove an electron from an atom is called the ionization energy. The first ionization energy refers to the energy needed to remove the first electron, the second ionization energy refers to removing the second electron, and so on. Generally, subsequent ionization energies are progressively higher, as it becomes increasingly difficult to remove electrons from an increasingly positively charged ion.
Properties of Cations
Cations exhibit several distinct properties that differentiate them from their neutral atom counterparts:
- Positive Charge: The defining characteristic of a cation is its positive electrical charge.
- Smaller Size: Compared to their neutral atoms, cations are generally smaller in size. This is because the loss of electrons reduces the electron-electron repulsion, allowing the remaining electrons to be drawn closer to the nucleus.
- Reactivity: Cations are highly reactive, particularly in aqueous solutions. Their positive charge allows them to readily interact with negatively charged ions (anions) and polar molecules.
- Conducting Properties: Cations contribute to the electrical conductivity of solutions and molten salts. Their mobility allows them to carry electric charge.
Importance of Cations in Various Fields
Cations play pivotal roles in a wide range of scientific disciplines and technological applications:
1. Chemistry:
- Chemical Reactions: Cations participate in countless chemical reactions, forming ionic compounds and influencing reaction mechanisms. For example, the reaction between sodium metal and chlorine gas to form sodium chloride (table salt) involves the formation of sodium cations (Na⁺) and chloride anions (Cl⁻).
- Electrochemistry: Cations are crucial in electrochemical processes, such as batteries and fuel cells. The movement of cations (and anions) across electrodes generates electrical current.
- Catalysis: Certain cations act as catalysts, accelerating the rate of chemical reactions without being consumed in the process.
2. Biology:
- Biological Processes: Many vital biological processes rely on the presence and movement of cations. For example, sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions are essential for nerve impulse transmission, muscle contraction, and enzyme activity. These cations are frequently transported across cell membranes through specialized channels and pumps, maintaining electrochemical gradients vital for life.
- Mineral Nutrition: Plants require a variety of cations, including potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), for their growth and development. These nutrients are absorbed from the soil through their root systems.
3. Materials Science:
- Ionic Compounds: Cations form a wide array of ionic compounds with anions, exhibiting a variety of properties depending on the constituent ions. These compounds find numerous applications, ranging from ceramics to fertilizers.
- Semiconductors: Certain cation-doped semiconductors exhibit unique electrical properties crucial for electronic devices.
- Metal Alloys: The presence of cations influences the properties of metal alloys, such as strength, hardness, and corrosion resistance.
4. Geology:
- Mineral Formation: Cations are fundamental components of many minerals found in the Earth's crust. The specific combination of cations and anions determines the mineral's crystal structure and properties.
- Geochemical Cycles: Cations play a significant role in geochemical cycles, such as the weathering of rocks and the transport of dissolved ions in rivers and oceans.
5. Medicine:
- Electrolyte Balance: Maintaining the proper balance of cations (and anions) in the body's fluids (electrolyte balance) is essential for health. Imbalances can lead to serious medical conditions.
- Drug Delivery: Certain cations are used in drug delivery systems to target specific cells or tissues.
Distinguishing Cations from Anions
It's important to distinguish cations from anions, which are atoms or groups of atoms with a negative charge. Anions are formed when a neutral atom gains one or more electrons. For example, a chlorine atom (Cl) gains one electron to become a chloride anion (Cl⁻). The opposite charges of cations and anions allow them to attract each other, forming ionic compounds through electrostatic interactions.
Conclusion: The Ubiquitous Role of Cations
In conclusion, a cation, an atom with a positive charge, is far from a simple concept. It represents a fundamental building block of chemistry and physics, playing a critical role in a vast array of processes and applications across various scientific disciplines. From driving chemical reactions and powering batteries to maintaining life itself, cations are integral to the world around us. A deeper understanding of cations provides crucial insights into the workings of nature and the development of advanced technologies. Further exploration into specific types of cations and their unique properties can unveil even more fascinating aspects of this essential part of the atomic world.
Latest Posts
Latest Posts
-
How Many Gallons In 4 5 Liters
May 09, 2025
-
A Gas Has No Definite Shape And Volume
May 09, 2025
-
What Is 0 63 Expressed As A Fraction In Simplest Form
May 09, 2025
-
360 Inches Is How Many Yards
May 09, 2025
-
Arrange These Elements According To Electronegativity
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is An Atom With A Positive Charge Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.