What Is A Property Of A Base
listenit
Mar 15, 2025 · 7 min read
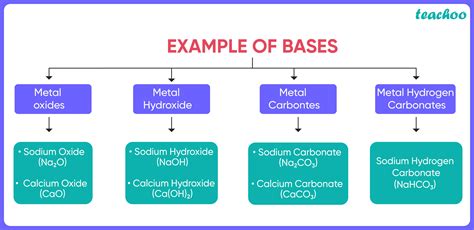
Table of Contents
What is a Property of a Base? A Comprehensive Guide
Understanding the properties of bases is fundamental to chemistry. Bases, along with acids, form the cornerstone of acid-base chemistry, a critical area impacting various fields, from industrial processes to biological systems. This comprehensive guide delves into the defining characteristics of bases, exploring their chemical properties, physical properties, and practical applications. We will also examine different definitions of bases and how these definitions relate to their properties.
Defining Bases: Arrhenius, Brønsted-Lowry, and Lewis
Before diving into the properties, it's crucial to establish a clear understanding of what constitutes a base. Several definitions exist, each offering a slightly different perspective:
1. Arrhenius Definition:
The Arrhenius definition, one of the earliest, defines a base as a substance that increases the hydroxide ion (OH⁻) concentration when dissolved in water. This definition is simple and directly relates to the production of hydroxide ions, which are responsible for the characteristic properties of alkaline solutions. However, it limits the scope of bases to aqueous solutions only. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH). These strong bases completely dissociate in water, releasing a high concentration of OH⁻ ions.
2. Brønsted-Lowry Definition:
A broader definition is provided by the Brønsted-Lowry theory. This theory defines a base as a proton acceptor. This definition is not restricted to aqueous solutions; it encompasses a wider range of reactions and substances. A Brønsted-Lowry base accepts a proton (H⁺) from an acid, forming its conjugate acid. For example, ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water to form ammonium ion (NH₄⁺) and hydroxide ion (OH⁻).
3. Lewis Definition:
The most comprehensive definition comes from the Lewis theory. This defines a base as an electron-pair donor. This definition is the most encompassing, extending beyond proton transfer reactions. A Lewis base donates a lone pair of electrons to a Lewis acid (an electron-pair acceptor), forming a coordinate covalent bond. Many compounds with lone pairs of electrons, such as ammonia (NH₃) and water (H₂O), can act as Lewis bases. This definition greatly expands the scope of base chemistry to include reactions that don't involve protons.
Chemical Properties of Bases
The chemical behavior of bases is largely determined by their ability to accept protons or donate electron pairs. Key chemical properties include:
1. Reaction with Acids: Neutralization
The most characteristic reaction of a base is its reaction with an acid, a process known as neutralization. This reaction leads to the formation of salt and water. The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H₂O):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
The heat released during this reaction is an indication of the strength of the acid-base interaction.
2. Reaction with Metals: Hydrogen Gas Evolution
Certain bases, particularly strong bases like alkali metal hydroxides, react with certain metals to produce hydrogen gas. This reaction is typically observed with amphoteric metals, which can react with both acids and bases. For example, the reaction between zinc (Zn) and sodium hydroxide (NaOH) produces sodium zincate and hydrogen gas:
Zn(s) + 2NaOH(aq) → Na₂ZnO₂(aq) + H₂(g)
3. Saponification: Soap Formation
Bases play a vital role in the saponification process, which is the formation of soap from fats and oils. Strong bases like sodium hydroxide (NaOH) and potassium hydroxide (KOH) hydrolyze fats and oils (esters of fatty acids) to produce glycerol and fatty acid salts, which are soaps.
4. Amide Formation: Reaction with Acid Chlorides and Anhydrides
Bases can react with acid chlorides and anhydrides to form amides. This reaction is a crucial step in many organic synthesis processes. The base typically deprotonates a nitrogen-containing compound, enabling it to act as a nucleophile and attack the carbonyl carbon of the acid chloride or anhydride.
Physical Properties of Bases
Beyond their chemical reactivity, bases exhibit specific physical properties that help identify them:
1. Taste: Bitter Taste
Bases typically have a bitter taste. However, it's crucial to never taste chemicals in a laboratory setting due to the potential dangers involved.
2. Feel: Slippery or Soapy Feel
Bases often feel slippery or soapy to the touch. This sensation is due to the reaction of the base with the oils and proteins on the skin, forming a soap-like substance.
3. pH: High pH Values
The most definitive physical property of bases is their high pH value. The pH scale measures the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is neutral; values below 7 are acidic, and values above 7 are alkaline (basic). Strong bases have pH values close to 14, while weak bases have pH values slightly above 7.
4. Conductivity: Electrical Conductivity
Aqueous solutions of bases are typically good conductors of electricity. This is because the dissolved base dissociates into ions (cations and hydroxide anions), which can carry an electric current. The stronger the base, the greater its conductivity.
5. Indicators: Color Change with Indicators
Several indicators change color depending on the pH of the solution. These indicators can be used to visually identify bases. For example, phenolphthalein is colorless in acidic solutions and turns pink in basic solutions. Litmus paper turns blue in the presence of bases.
Strength of Bases: Strong vs. Weak Bases
Bases are classified as either strong or weak, depending on their degree of dissociation in water:
Strong bases completely dissociate in water, releasing all their hydroxide ions. Examples include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)₂)
- Barium hydroxide (Ba(OH)₂)
Weak bases only partially dissociate in water, meaning only a small fraction of the base molecules release hydroxide ions. Examples include:
- Ammonia (NH₃)
- Pyridine (C₅H₅N)
- Methylamine (CH₃NH₂)
Applications of Bases
The diverse properties of bases make them essential in a wide range of applications:
1. Industrial Applications:
- Manufacturing: Bases are crucial in the production of various materials, including soaps, detergents, fertilizers, and plastics.
- Pulp and Paper Industry: Bases are used in the pulping process to separate cellulose fibers from wood.
- Water Treatment: Bases help adjust the pH of water, making it suitable for drinking and industrial purposes.
2. Biological Applications:
- pH Regulation: Bases play a crucial role in maintaining the pH balance in biological systems, such as blood. Buffers, which contain weak acids and their conjugate bases, are essential in this process.
- Enzyme Activity: Many enzymes require specific pH ranges for optimal activity, and bases can help maintain these conditions.
- Digestion: The digestive system utilizes bases to neutralize stomach acid.
3. Household Applications:
- Cleaning Products: Many household cleaning products contain bases, such as drain cleaners and oven cleaners, due to their ability to dissolve grease and other substances.
- Baking: Baking soda (sodium bicarbonate), a weak base, is used as a leavening agent in baking.
Safety Precautions when Handling Bases
Strong bases are corrosive and can cause severe burns to the skin and eyes. When handling bases, it's essential to follow these safety precautions:
- Wear appropriate safety equipment: Always wear safety goggles, gloves, and a lab coat when handling bases.
- Handle with care: Avoid direct contact with skin and eyes.
- Neutralize spills immediately: If a base is spilled, neutralize it immediately with a weak acid, such as vinegar, and clean up the area thoroughly.
- Proper Disposal: Dispose of base solutions according to your institution's guidelines.
Conclusion
Bases are a fundamental class of chemical compounds with diverse properties and applications. Understanding their chemical and physical properties, their different definitions, and their strengths is vital in various scientific and industrial fields. Always remember to prioritize safety when working with bases, as improper handling can lead to hazardous consequences. This comprehensive guide has provided a strong foundation for understanding this essential aspect of chemistry. Further exploration into specific types of bases and their detailed reactions will deepen your understanding of their significance in the broader chemical landscape.
Latest Posts
Latest Posts
-
Do Plant Cells Have A Mitochondria
Mar 15, 2025
-
What Percent Of 40 Is 80
Mar 15, 2025
-
70 Of What Number Is 35
Mar 15, 2025
-
Words That Start With Same Letter
Mar 15, 2025
-
60 Miles Per Hour Is How Many Feet Per Second
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about What Is A Property Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
