What Is A Column On The Periodic Table Called
listenit
Mar 14, 2025 · 7 min read
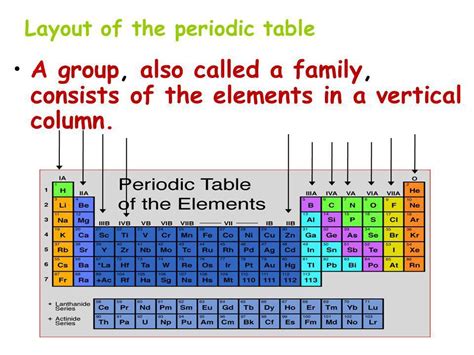
Table of Contents
What is a Column on the Periodic Table Called? Understanding Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While rows are known as periods, columns hold a different, equally important designation: groups. Understanding the difference between periods and groups, and the significance of group properties, is crucial for comprehending chemical behavior and predicting reactions. This comprehensive guide delves deep into the concept of groups on the periodic table, exploring their characteristics, trends, and practical applications.
Defining Groups on the Periodic Table: A Vertical Arrangement of Elements
A group, also known as a family, represents a vertical column on the periodic table. Elements within the same group share similar chemical properties due to their identical number of valence electrons. Valence electrons are the electrons in the outermost shell of an atom, and they are the primary participants in chemical bonding. This shared characteristic leads to predictable patterns in reactivity, bonding preferences, and other chemical behaviors.
The Significance of Valence Electrons
The significance of valence electrons cannot be overstated. These electrons determine how an element will interact with other elements. Elements in the same group have the same number of valence electrons, leading to similar chemical behaviors. For instance, alkali metals (Group 1) all have one valence electron, making them highly reactive and prone to losing that electron to form +1 ions. Similarly, halogens (Group 17) have seven valence electrons, making them eager to gain one electron to achieve a stable octet and form -1 ions.
Exploring the Major Groups on the Periodic Table
The periodic table boasts eighteen groups, each with unique properties and characteristics. While each group possesses its own distinct features, some overarching trends and relationships exist across the table. Let's examine some of the prominent groups:
Group 1: Alkali Metals
The alkali metals, comprising lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are highly reactive metals. Their single valence electron readily participates in ionic bonding, forming +1 ions. This reactivity increases as you move down the group. Alkali metals are soft, have low melting points, and readily react with water, producing hydrogen gas. Their compounds are widely used in various applications, from table salt (sodium chloride) to specialized alloys and batteries.
Group 2: Alkaline Earth Metals
Alkaline earth metals (beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra)) are less reactive than alkali metals, possessing two valence electrons. They tend to form +2 ions. These metals are also relatively soft, but they have higher melting points than alkali metals. Alkaline earth metals play vital roles in biological processes (calcium in bones, magnesium in chlorophyll) and are used extensively in construction materials (cement, plaster) and industrial applications (magnesium alloys).
Group 17: Halogens
Halogens (fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At)) are highly reactive nonmetals. With seven valence electrons, they readily gain one electron to form -1 ions, achieving a stable electron configuration. Their reactivity decreases down the group, with fluorine being the most reactive halogen. Halogens are used in various applications, from disinfectants (chlorine) to refrigerants (fluorocarbons) and essential components in many industrial processes. However, caution is necessary as many halogens and their compounds are toxic.
Group 18: Noble Gases
Noble gases (helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)) are inert, unreactive elements. They possess a full valence shell (eight electrons, except for helium with two), rendering them chemically stable. Their lack of reactivity makes them valuable in various applications, including lighting (neon lights), welding (argon), and medical imaging (xenon).
Beyond the Main Groups: Transition Metals and Inner Transition Metals
The periodic table also includes transition metals and inner transition metals, adding complexity and richness to the properties of elements.
Transition Metals: A Rich Tapestry of Properties
Transition metals occupy the d-block of the periodic table, characterized by partially filled d orbitals. Their valence electrons are more varied than those in main group elements, leading to a wider range of oxidation states and complex chemical behavior. Transition metals exhibit catalytic properties (used in many industrial processes), form brightly colored compounds, and possess magnetic properties (ferromagnetism in iron, cobalt, and nickel). These metals are widely used in various applications, including alloys (steel, bronze), catalysts, pigments, and electronic components. The versatility of their properties makes them crucial in numerous industrial and technological applications.
Inner Transition Metals: The Lanthanides and Actinides
The inner transition metals, also known as the f-block elements, are divided into lanthanides and actinides. These elements have partially filled f orbitals, leading to unique chemical and physical properties. Lanthanides are used in various applications, including lighting (lanthanum in high-intensity lamps) and alloys. Actinides, including uranium and plutonium, are known for their radioactive properties and are used in nuclear energy and research. However, they also pose significant health risks due to their radioactivity.
Trends within Groups: Atomic Radius, Ionization Energy, and Electronegativity
Understanding trends in group properties is crucial for predicting chemical behavior. Several key properties exhibit predictable patterns within groups:
Atomic Radius: Increasing Down a Group
Atomic radius, the size of an atom, generally increases as you move down a group. This is because additional electron shells are added, increasing the distance between the nucleus and the outermost electrons. This increase in atomic radius influences the reactivity and bonding characteristics of elements within a group.
Ionization Energy: Decreasing Down a Group
Ionization energy, the energy required to remove an electron from an atom, generally decreases as you move down a group. The increasing atomic radius and shielding effect of inner electrons make it easier to remove an electron from larger atoms. This trend influences the ease with which elements form ions and participate in chemical reactions.
Electronegativity: Decreasing Down a Group
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally decreases as you move down a group. The increasing atomic radius reduces the attraction between the nucleus and the outermost electrons, making it less likely to attract electrons from other atoms. This impacts the type of bonds formed and the polarity of molecules.
Applications and Importance of Understanding Groups
Understanding the properties of groups on the periodic table is fundamental to various aspects of chemistry and related fields:
- Predicting Chemical Reactions: Knowing the group an element belongs to helps predict its reactivity and how it will interact with other elements.
- Designing New Materials: The properties of elements in specific groups are crucial in designing new materials with tailored characteristics.
- Developing New Technologies: Understanding group trends is vital in developing new technologies, from advanced batteries to catalysts for sustainable energy production.
- Environmental Science: Knowledge of group properties is essential in understanding the environmental impact of elements and their compounds.
- Biological Sciences: The role of specific elements in biological systems is directly related to their group properties and their impact on biochemical processes.
Conclusion: Groups as the Key to Understanding Chemical Behavior
The columns on the periodic table, known as groups or families, are not just a convenient organizational feature. They are a powerful tool for understanding chemical behavior. The shared number of valence electrons within a group leads to predictable trends in properties like reactivity, bonding, and ionization energy. Understanding these trends allows chemists to predict the behavior of elements, design new materials, and develop new technologies. The organization of the periodic table by groups is a testament to the underlying order and predictability within the vast and complex world of chemistry. From the highly reactive alkali metals to the inert noble gases, each group tells a unique story, contributing to our comprehensive understanding of the elements and their interactions. Further exploration into specific groups and their individual properties can unlock deeper insights into the intricate workings of the chemical world.
Latest Posts
Latest Posts
-
Ln X 1 Ln X 1
Mar 14, 2025
-
P 2l 2w Solve For W
Mar 14, 2025
-
What Percent Is 28 Of 40
Mar 14, 2025
-
2x 3 X 4 2 3
Mar 14, 2025
-
What Percent Is 12 Of 60
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Is A Column On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
