What Is A Column Called In The Periodic Table
listenit
Mar 16, 2025 · 6 min read
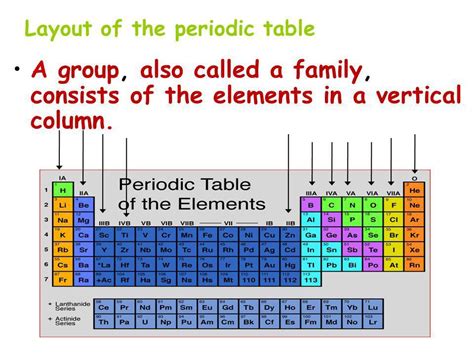
Table of Contents
What is a Column Called in the Periodic Table? Understanding Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. Understanding its structure is crucial for comprehending the behavior of matter. One of the fundamental organizational principles is the arrangement of elements into columns, which are formally known as groups. This article delves deep into what these columns are called, the significance of their arrangement, and the trends in properties they exhibit.
Understanding the Periodic Table's Structure: Rows and Columns
The periodic table arranges elements in a grid, featuring both rows and columns. The rows, known as periods, represent increasing atomic number and, consequently, the addition of electron shells. As you move across a period, the number of protons and electrons increases, leading to changes in chemical properties.
The columns, or groups, represent elements with similar outer electron configurations. This shared outer electron configuration dictates their chemical behavior, leading to similar reactivity and bonding patterns. This is why understanding what a column is called in the periodic table—a group—is so critical to understanding chemistry.
What are Groups in the Periodic Table?
Groups, the columns in the periodic table, are families of elements sharing similar chemical properties. These similarities stem from the same number of valence electrons—the electrons in the outermost shell. Valence electrons participate in chemical bonding, largely determining how an element reacts with other elements. Elements within the same group tend to exhibit similar reactivity, forming similar types of compounds.
The Significance of Valence Electrons
The number of valence electrons directly influences an element's chemical behavior. For example, elements in Group 1 (alkali metals) have one valence electron, making them highly reactive and readily losing that electron to form +1 ions. Elements in Group 17 (halogens) have seven valence electrons, readily gaining one electron to form -1 ions. This consistent pattern across groups highlights the importance of the column's arrangement in the periodic table.
Naming Conventions for Groups
The groups in the periodic table are numbered in two main systems:
1. The IUPAC Numbering System: A Universal Standard
The International Union of Pure and Applied Chemistry (IUPAC) recommends a simple numbering system, using Arabic numerals 1 through 18, running from left to right across the table. This system provides a clear and unambiguous way to identify each group. For instance, Group 1 represents the alkali metals, Group 17 the halogens, and Group 18 the noble gases. This universal standard is widely accepted and promotes clarity in chemical communication.
2. The Older Group Numbering System: Roman Numerals and 'A' and 'B' Designations
Older literature often uses a system employing Roman numerals (I to VIII) and the letters 'A' and 'B'. This system was less consistent and created ambiguities, particularly in the transition metal region. While still encountered, the IUPAC numbering system is now preferred for its clarity and universal adoption. Understanding both systems helps interpret older textbooks and research papers.
Exploring Key Groups and Their Properties: A Detailed Overview
Let's explore some key groups, highlighting their characteristics and the significance of their column placement:
Group 1: Alkali Metals
The alkali metals (Li, Na, K, Rb, Cs, Fr) are highly reactive metals characterized by their single valence electron. This electron is easily lost, forming +1 ions and resulting in high reactivity with water and oxygen. Their reactivity increases as you move down the group due to increasing atomic size and decreasing ionization energy.
- Key Properties: Soft, low density, low melting points, highly reactive.
- Chemical Behavior: Form +1 ions, readily react with nonmetals, particularly halogens.
Group 2: Alkaline Earth Metals
Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) possess two valence electrons, leading to a slightly lower reactivity compared to alkali metals. They readily lose their two valence electrons to form +2 ions. Their properties show similar trends to alkali metals, but with generally higher melting and boiling points.
- Key Properties: Harder than alkali metals, higher melting and boiling points, reactive but less so than alkali metals.
- Chemical Behavior: Form +2 ions, react with water (less vigorously than alkali metals), readily react with oxygen.
Group 17: Halogens
Halogens (F, Cl, Br, I, At) are highly reactive nonmetals with seven valence electrons. They readily gain one electron to achieve a stable octet, forming -1 ions. Their reactivity decreases as you move down the group due to increasing atomic size.
- Key Properties: Highly reactive nonmetals, various physical states (gas, liquid, solid), form diatomic molecules (e.g., Cl₂).
- Chemical Behavior: Form -1 ions, react readily with metals to form salts (e.g., NaCl – sodium chloride).
Group 18: Noble Gases
Noble gases (He, Ne, Ar, Kr, Xe, Rn) are inert gases with a full valence shell (eight electrons, except for helium with two). This complete electron configuration renders them exceptionally stable and unreactive, rarely forming compounds.
- Key Properties: Inert, colorless, odorless gases, low boiling points.
- Chemical Behavior: Extremely low reactivity, historically considered chemically inert although some compounds of heavier noble gases have been synthesized.
Transition Metals: A Unique Block
Transition metals occupy the central block of the periodic table, exhibiting a range of oxidation states and forming colorful compounds. They have variable valence electrons and often participate in complex ion formation. Their properties are less predictable compared to main group elements. This unique behavior arises from their partially filled d orbitals.
- Key Properties: Variable oxidation states, often form colored compounds, good conductors of electricity, high melting points.
- Chemical Behavior: Can form a variety of compounds with different oxidation states, catalyze many chemical reactions.
Trends within Groups: Predicting Properties
The periodic table allows us to predict the properties of elements based on their group and position. Several key trends are observed within groups:
- Atomic Radius: Generally increases as you move down a group due to the addition of electron shells.
- Ionization Energy: Generally decreases as you move down a group because the outermost electron is farther from the nucleus and less strongly attracted.
- Electronegativity: Generally decreases as you move down a group, reflecting the weaker attraction for electrons by larger atoms.
- Reactivity: Reactivity trends vary depending on the group. For example, alkali metals become more reactive going down the group, while halogens become less reactive.
Applications of Group Understanding
Understanding the properties of elements within groups has wide-ranging applications:
- Material Science: Designing new materials with specific properties often involves selecting elements from particular groups.
- Chemical Synthesis: Predicting reactivity helps chemists design efficient synthetic routes for new compounds.
- Environmental Chemistry: Understanding the behavior of elements in the environment, such as the reactivity of pollutants, relies heavily on periodic table knowledge.
- Biological Chemistry: Trace elements crucial for biological functions often belong to specific groups, and understanding their roles requires knowledge of their chemical properties.
Conclusion: The Importance of Group Classification
In conclusion, understanding what a column is called in the periodic table—a group—is paramount in chemistry. The systematic organization of elements into groups based on their electron configurations allows for prediction of their properties, facilitating the understanding of chemical reactions and the design of new materials. Whether employing the IUPAC numbering system or encountering the older Roman numeral notation, recognizing the significance of group classification is essential for any aspiring or practicing chemist. The consistent trends observed within groups provide a powerful framework for comprehending the behavior of matter and predicting its properties, making the periodic table an indispensable tool in scientific investigation.
Latest Posts
Latest Posts
-
Translating Graph Up By 4 Units
Mar 17, 2025
-
What Is The Least Common Multiple Of 3 2
Mar 17, 2025
-
What Is The Derivative Of Ln 1 X
Mar 17, 2025
-
Which Type Of Molecule Is Shown Below
Mar 17, 2025
-
How To Find The Domain Of F O G
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is A Column Called In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
