What Happens To The Temperature During A Phase Change
listenit
Apr 03, 2025 · 5 min read
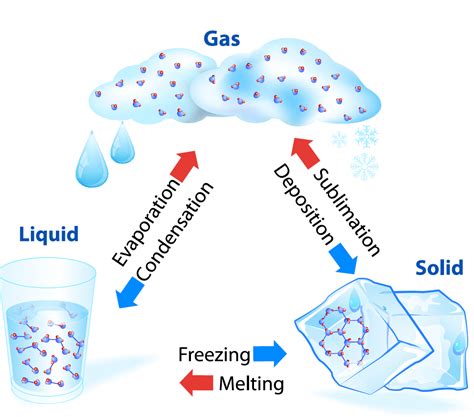
Table of Contents
- What Happens To The Temperature During A Phase Change
- Table of Contents
- What Happens to Temperature During a Phase Change?
- The Role of Latent Heat
- Understanding the Energy Transfer
- The Phase Change Process: A Detailed Look
- 1. Heating the Ice (Solid Phase):
- 2. Melting the Ice (Phase Transition: Solid to Liquid):
- 3. Heating the Water (Liquid Phase):
- 4. Boiling the Water (Phase Transition: Liquid to Gas):
- 5. Heating the Steam (Gas Phase):
- Factors Affecting Phase Transitions
- Practical Applications of Latent Heat
- Conclusion: The Constant Temperature Paradox
- Latest Posts
- Latest Posts
- Related Post
What Happens to Temperature During a Phase Change?
Understanding phase transitions is crucial in various fields, from meteorology and material science to cooking and even cryogenics. A phase change refers to the transformation of a substance from one state of matter to another, such as from solid to liquid (melting), liquid to gas (boiling/vaporization), or solid to gas (sublimation). A key characteristic of these transitions is the intriguing behavior of temperature. Contrary to what many might initially assume, temperature doesn't necessarily change continuously during a phase change. Let's delve into the details.
The Role of Latent Heat
The key to understanding temperature behavior during phase transitions lies in the concept of latent heat. Latent heat is the energy absorbed or released during a phase change at a constant temperature. This energy is not used to raise the temperature of the substance, but rather to overcome the intermolecular forces holding the substance in its current phase. Think of it like this: breaking bonds requires energy, even if the substance remains at the same temperature.
There are different types of latent heat:
- Latent heat of fusion: The energy required to change a substance from a solid to a liquid at its melting point (or released when freezing).
- Latent heat of vaporization: The energy required to change a substance from a liquid to a gas at its boiling point (or released when condensing).
- Latent heat of sublimation: The energy required to change a substance from a solid to a gas directly, without passing through the liquid phase (or released when depositing).
Understanding the Energy Transfer
When heat is added to a substance, the energy is initially used to increase the kinetic energy of its molecules, resulting in a temperature increase. However, once the substance reaches its phase transition point (melting point, boiling point, etc.), the added heat is no longer used to raise the temperature. Instead, it's used to overcome the intermolecular forces holding the molecules in their current arrangement. This means that the temperature remains constant during the phase change, even though energy is continuously being added.
Similarly, when a substance changes from a higher-energy phase to a lower-energy phase (e.g., liquid to solid), latent heat is released. This released energy compensates for the energy lost during the phase transition, keeping the temperature constant during the change.
The Phase Change Process: A Detailed Look
Let's illustrate this with a specific example: heating ice to steam.
1. Heating the Ice (Solid Phase):
Initially, as heat is added to ice, its temperature increases linearly. The added energy increases the kinetic energy of the water molecules within the ice crystal lattice. This increase in kinetic energy manifests as a rise in temperature. This continues until the ice reaches its melting point (0°C at standard atmospheric pressure).
2. Melting the Ice (Phase Transition: Solid to Liquid):
At 0°C, the added heat is no longer used to increase the temperature. Instead, it's used to break the hydrogen bonds holding the water molecules in the ice lattice. This process requires a significant amount of energy – the latent heat of fusion. During this phase change, the temperature remains constant at 0°C even though heat is continuously being added. The ice gradually melts into liquid water.
3. Heating the Water (Liquid Phase):
Once all the ice has melted, the added heat once again increases the kinetic energy of the water molecules, resulting in a temperature increase. The temperature rises linearly until it reaches the boiling point (100°C at standard atmospheric pressure).
4. Boiling the Water (Phase Transition: Liquid to Gas):
At 100°C, the added heat is once again used to overcome intermolecular forces, this time to transition the liquid water into water vapor (steam). This requires the latent heat of vaporization, a considerably larger amount of energy than the latent heat of fusion. The temperature remains constant at 100°C during this phase transition, despite the continuous addition of heat. The liquid water gradually boils and turns into steam.
5. Heating the Steam (Gas Phase):
After all the water has vaporized, the added heat once again increases the kinetic energy of the water molecules in the gaseous phase, resulting in a further temperature increase of the steam.
Factors Affecting Phase Transitions
Several factors can influence the temperature during phase transitions:
- Pressure: Changing the pressure on a substance can alter its melting and boiling points. Higher pressure generally increases the boiling point and may slightly decrease the melting point (water is a notable exception).
- Impurities: The presence of impurities in a substance can also affect its melting and boiling points. Impurities often depress the freezing point and elevate the boiling point.
- Rate of Heating/Cooling: The rate at which heat is added or removed can affect how long a phase transition takes, but it doesn't change the temperature during the transition itself.
Practical Applications of Latent Heat
The concept of latent heat has numerous practical applications:
- Cooling Systems: Refrigeration and air conditioning systems utilize the latent heat of vaporization of refrigerants to absorb heat from their surroundings.
- Heating Systems: Steam heating systems take advantage of the latent heat of condensation to release heat into a building.
- Cooking: Cooking processes often involve phase transitions, and understanding latent heat is crucial for achieving the desired results.
- Material Processing: Many industrial processes involve the melting and solidification of materials, and a thorough understanding of latent heat is essential for controlling these processes.
- Weather Phenomena: Weather patterns are heavily influenced by phase transitions of water, such as cloud formation (condensation) and snow/rain formation (freezing/melting).
Conclusion: The Constant Temperature Paradox
The constant temperature maintained during phase transitions might seem paradoxical at first. We're accustomed to adding heat resulting in a temperature rise. However, during phase transitions, the added energy is used to break intermolecular forces rather than increasing the kinetic energy of the molecules. This crucial distinction explains why temperature remains constant despite the continuous energy input or output. Understanding this principle is fundamental to appreciating the behavior of matter in various states and its diverse applications in different fields. By grasping the concept of latent heat, we gain a deeper understanding of the physical world around us. From everyday cooking to complex industrial processes, the principles of phase transitions and latent heat play a vital role.
Latest Posts
Latest Posts
-
Is The Nucleus The Brain Of The Cell
Apr 07, 2025
-
What Are The Subunits Called That Make Up Carbohydrates
Apr 07, 2025
-
What Is The Earths Only Liquid Layer
Apr 07, 2025
-
How Many Miles In 1000 Feet
Apr 07, 2025
-
What Is 2 And 5 8 As A Decimal
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about What Happens To The Temperature During A Phase Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
