What Happens To The Atoms In A Chemical Reaction
listenit
Mar 19, 2025 · 6 min read
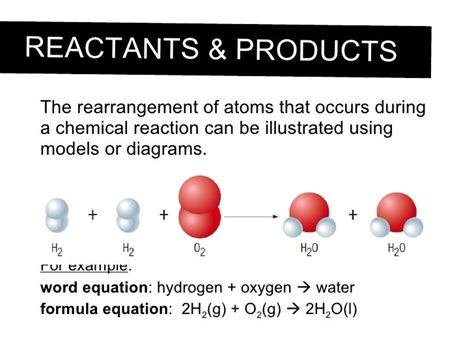
Table of Contents
What Happens to the Atoms in a Chemical Reaction?
Chemical reactions are the fundamental processes that govern the world around us, from the rusting of iron to the photosynthesis in plants. But what exactly happens to the atoms involved in these transformations? Understanding this is key to grasping the essence of chemistry. This article delves deep into the fascinating world of atomic behavior during chemical reactions, exploring concepts like conservation of mass, rearrangement of atoms, and the role of energy.
The Law of Conservation of Mass: Atoms are Neither Created Nor Destroyed
A cornerstone of chemistry is the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. This means that the total mass of the reactants (the starting materials) must equal the total mass of the products (the resulting substances). While the substances themselves change dramatically, the atoms involved remain intact. They simply rearrange themselves to form new molecules.
Think of it like building with LEGO bricks. You can take apart a spaceship and rebuild it into a castle, but the number of bricks remains the same. Similarly, in a chemical reaction, the atoms are the "bricks," and they are rearranged to form different structures, but their total number remains constant.
Example: The Burning of Methane
Let's consider the combustion of methane (CH₄), a simple hydrocarbon, as an example. The balanced chemical equation is:
CH₄ + 2O₂ → CO₂ + 2H₂O
This equation tells us that one molecule of methane reacts with two molecules of oxygen (O₂) to produce one molecule of carbon dioxide (CO₂) and two molecules of water (H₂O). Let's count the atoms:
- Reactants: 1 carbon atom, 4 hydrogen atoms, 4 oxygen atoms
- Products: 1 carbon atom, 4 hydrogen atoms, 4 oxygen atoms
Notice that the number of each type of atom is identical on both sides of the equation. The atoms have simply rearranged themselves, forming new chemical bonds. The carbon atom from methane is now bonded to two oxygen atoms in carbon dioxide, and the hydrogen atoms are now bonded to oxygen atoms in water molecules. No atoms have been lost or gained; the mass remains constant.
Rearrangement of Atoms: Breaking and Forming Chemical Bonds
The essence of a chemical reaction lies in the rearrangement of atoms, which involves the breaking and forming of chemical bonds. Chemical bonds are the forces that hold atoms together in molecules. These bonds are formed by the sharing or transfer of electrons between atoms.
Breaking Bonds: Requires Energy
Breaking existing chemical bonds requires energy. This energy is often supplied in the form of heat, light, or electricity. The energy needed to break bonds is called the bond energy. The stronger the bond, the more energy is required to break it.
Forming Bonds: Releases Energy
When new chemical bonds are formed, energy is released. This energy is often released as heat, light, or other forms of energy. The energy released during bond formation is also related to the strength of the bonds. Stronger bonds release more energy upon formation.
The Energy Balance: Exothermic and Endothermic Reactions
The overall energy change in a chemical reaction is the difference between the energy required to break bonds and the energy released when new bonds are formed. If more energy is released than is required, the reaction is exothermic, meaning it releases heat to the surroundings. If more energy is required than is released, the reaction is endothermic, meaning it absorbs heat from the surroundings.
The Role of Activation Energy: Getting the Reaction Started
Even if a reaction is exothermic (meaning it releases energy overall), it still requires an initial input of energy to get started. This initial energy is called the activation energy. The activation energy is like the push needed to get a boulder rolling down a hill. Once the boulder starts rolling, it releases energy as it descends, but it still needed that initial push.
The activation energy is used to break the initial chemical bonds, allowing the reaction to proceed. Once the reaction is underway, the energy released from forming new bonds can sustain the reaction, provided the energy released exceeds the energy required for activation.
Factors Affecting Reaction Rates: Speeding Up or Slowing Down
Several factors influence the rate of a chemical reaction:
- Temperature: Higher temperatures generally increase the rate of reaction by providing more energy for molecules to overcome the activation energy barrier.
- Concentration: A higher concentration of reactants leads to more frequent collisions between reactant molecules, increasing the reaction rate.
- Surface Area: For reactions involving solids, a larger surface area increases the contact between reactants, thus increasing the reaction rate.
- Presence of a Catalyst: Catalysts are substances that speed up the rate of a reaction without being consumed themselves. They lower the activation energy, making it easier for the reaction to occur.
Beyond Simple Reactions: Complex Chemical Processes
While the principles discussed above apply to all chemical reactions, many reactions are far more complex than the simple example of methane combustion. In complex reactions, multiple steps may be involved, with intermediate products formed and consumed along the way. Understanding these complex reaction mechanisms often requires advanced chemical techniques and modeling.
Advanced Concepts: Isotopes and Nuclear Reactions
The discussion so far has focused on chemical reactions, which involve the rearrangement of atoms without changing the nuclei of the atoms. However, it is important to note that isotopes, atoms of the same element with differing numbers of neutrons, behave similarly in chemical reactions. The number of protons defines the element, while neutrons contribute to mass but don’t affect chemical reactivity in a significant way.
Nuclear reactions, on the other hand, involve changes in the nuclei of atoms. These reactions are fundamentally different from chemical reactions and often involve immense energy changes. Nuclear reactions are not governed by the simple law of conservation of mass in the same way, as some mass is converted to energy according to Einstein's famous equation, E=mc².
Conclusion: The Ever-Changing Dance of Atoms
Chemical reactions are a dynamic interplay of atoms, bonds, and energy. The fundamental principle of conservation of mass ensures that atoms are neither created nor destroyed, but rather rearranged into new configurations. Understanding how atoms behave during these reactions is fundamental to comprehending the vast range of chemical processes that shape our world, from the simplest biological processes to the most complex industrial technologies. The detailed study of bond energies, activation energies, and reaction rates allows us to predict, control, and utilize chemical transformations for numerous applications. The field of chemistry continues to unravel the intricate dance of atoms, revealing ever-deeper insights into the fundamental nature of matter and its transformations.
Latest Posts
Latest Posts
-
What Is Square Root Of 52
Mar 19, 2025
-
Why Do Plants Need Both Chloroplasts And Mitochondria
Mar 19, 2025
-
What Percentage Is 1 Of 7
Mar 19, 2025
-
What Percentage Of 38 Is 15
Mar 19, 2025
-
How Many Feet Is 240 In
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Happens To The Atoms In A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
