What Do Brackets Mean In Chemistry
listenit
Mar 14, 2025 · 6 min read
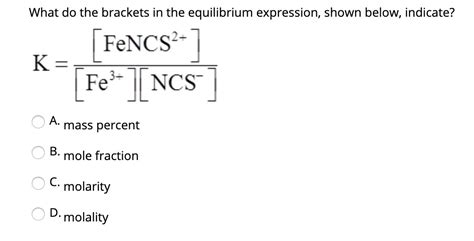
Table of Contents
What Do Brackets Mean in Chemistry? A Comprehensive Guide
Brackets in chemistry aren't just punctuation; they hold significant meaning, conveying crucial information about the structure, behavior, and context of chemical species. Understanding their various uses is essential for interpreting chemical formulas, equations, and reactions accurately. This comprehensive guide will explore the multifaceted roles of brackets in the chemical world, clarifying their usage in different scenarios and providing examples for better comprehension.
Brackets Indicating Concentration in Chemistry
One of the most frequent uses of brackets in chemistry is to denote concentration. Specifically, square brackets, [ ], are universally used to represent the molar concentration of a substance in a solution. Molar concentration, often expressed as molarity (M), signifies the number of moles of a solute dissolved per liter of solution.
For instance, [NaCl] = 0.1 M indicates that the molar concentration of sodium chloride (NaCl) in the solution is 0.1 moles per liter. This notation is particularly vital in equilibrium calculations, reaction kinetics, and discussions regarding solution chemistry.
Applications of Concentration Notation
-
Equilibrium expressions: Brackets are fundamental in writing equilibrium constant expressions (K<sub>c</sub> or K<sub>eq</sub>). The expression shows the ratio of product concentrations to reactant concentrations, each raised to the power of its stoichiometric coefficient. For example, the equilibrium constant for the reaction aA + bB ⇌ cC + dD is expressed as: K<sub>c</sub> = [C]<sup>c</sup>[D]<sup>d</sup> / [A]<sup>a</sup>[B]<sup>b</sup>
-
Rate laws: In chemical kinetics, brackets denote the concentration of reactants in the rate law expression, which describes the relationship between the rate of a reaction and the concentration of the reactants. For example, a rate law might be expressed as: Rate = k[A][B], where k is the rate constant.
-
Solubility product: For sparingly soluble ionic compounds, the solubility product constant (K<sub>sp</sub>) uses brackets to represent the ion concentrations at equilibrium. For example, for the dissolution of AgCl: AgCl(s) ⇌ Ag<sup>+</sup>(aq) + Cl<sup>-</sup>(aq), the solubility product is written as K<sub>sp</sub> = [Ag<sup>+</sup>][Cl<sup>-</sup>].
Brackets in Coordination Compounds (Complex Ions)
In coordination chemistry, square brackets are crucial for representing coordination complexes, which are formed by a central metal ion surrounded by ligands (molecules or ions bound to the metal). The brackets enclose the metal ion and its directly bound ligands. This clearly distinguishes the coordination sphere from any counterions present.
For example, in [Fe(H<sub>2</sub>O)<sub>6</sub>]<sup>3+</sup>, the brackets enclose the central iron(III) ion (Fe<sup>3+</sup>) coordinated to six water molecules (H<sub>2</sub>O). The superscript 3+ indicates the overall charge of the complex ion. The counterions, if any, would be written outside the brackets. For instance, the compound potassium hexaaquairon(III) chloride would be represented as [Fe(H<sub>2</sub>O)<sub>6</sub>]Cl<sub>3</sub>.
Importance of Bracket Usage in Coordination Compounds
Using brackets correctly is vital to avoid ambiguity. Without brackets, the chemical formula could be misinterpreted. Correct notation helps to clarify the structure of the complex ion and its interaction with other species.
The brackets also emphasize the different bonding situations within the complex. The ligands inside the brackets are directly bonded to the central metal ion through coordinate covalent bonds. Any counterions outside the brackets are ionically bonded to the complex ion.
Brackets in Organic Chemistry: Functional Groups & Reactions
While less frequent than in inorganic or physical chemistry, brackets occasionally appear in organic chemistry, primarily in two contexts:
1. Representing Functional Groups within a Larger Molecule
Sometimes, to emphasize a specific functional group within a larger organic molecule, brackets might be used. This is less common and largely depends on the author's style or to highlight a particular aspect of the molecule for discussion. It's not a standard convention like in inorganic chemistry.
2. Representing Reaction Intermediates or Transition States
Brackets may be used informally to represent reaction intermediates or transition states that exist temporarily during a chemical reaction. These aren't stable species and typically aren't part of a formal chemical equation. Instead, they are often used in reaction mechanisms to visualize the step-by-step process. However, this is not a strictly standardized use and may vary based on author preference.
Curly Brackets { } and Parentheses ( ) in Chemistry
While square brackets [ ] have established meanings, curly brackets { } and parentheses ( ) have less defined roles in standard chemical notation. Their usage is usually context-dependent and less formally standardized.
Curly Brackets { }
Curly brackets are sometimes used to represent sets or collections of atoms or molecules. For example, they might be used in statistical mechanics or when discussing various isomers or conformers. However, their usage isn’t widespread or rigorously defined within the mainstream chemical notation.
Parentheses ( )
Parentheses are most commonly found in chemical formulas to indicate the number of specific groups or units within a molecule or complex. For example, in (CH<sub>3</sub>)<sub>2</sub>CHOH, the parentheses indicate that two methyl groups (CH<sub>3</sub>) are attached to the same carbon atom. They help to clarify the branching and overall structure of organic compounds. They're also used in naming complex compounds, helping to differentiate between various ligands or units.
Distinction and Importance of Correct Bracket Usage
It's crucial to distinguish between the various types of brackets and their specific meanings. Incorrect usage can lead to misinterpretations of chemical formulas, equations, and reaction mechanisms. Accuracy in chemical notation is paramount for clear communication and proper interpretation of chemical data.
The specific meaning of the brackets often depends on the context. However, the widespread use of square brackets for molar concentration and in coordination compounds makes understanding their roles indispensable for anyone involved in chemistry, whether at the introductory or advanced level.
Beyond the Basics: Advanced Applications of Brackets
The usage of brackets extends beyond the fundamental applications discussed above. In more advanced fields of chemistry, such as:
-
Spectroscopy: Brackets may be used to denote specific transitions or energy levels in spectroscopic analyses.
-
Quantum Chemistry: Brackets can appear in wave functions and other mathematical representations used in theoretical chemistry calculations.
-
Polymer Chemistry: Brackets might be used to represent repeating units in polymer structures.
These uses are often highly specialized and require a deeper understanding of the specific subfield of chemistry.
Conclusion: Mastering the Language of Chemistry
Brackets are fundamental elements of chemical notation, playing significant roles in representing concentration, coordination complexes, and conveying crucial information about chemical species. Understanding their different meanings is vital for accurately interpreting chemical formulas, equations, and reaction mechanisms. This guide has provided a comprehensive overview of bracket usage, highlighting their significance across various aspects of chemistry. As you progress in your chemical studies, paying attention to the nuances of bracket usage will become increasingly important for clear communication and a deeper grasp of the chemical world. By mastering this seemingly small aspect of chemical language, you significantly enhance your ability to understand and interpret chemical information effectively.
Latest Posts
Latest Posts
-
How Many Moles Of Ions Are In Of
Mar 14, 2025
-
What Is The Difference Between A Coefficient And A Subscript
Mar 14, 2025
-
What Is The Least Common Factor Of 9 And 15
Mar 14, 2025
-
What Is The Correct Equation For Cellular Respiration
Mar 14, 2025
-
How Many Pounds Is 1 2 Kg
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Do Brackets Mean In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
