What Color Of Light Has The Most Energy
listenit
Mar 14, 2025 · 5 min read
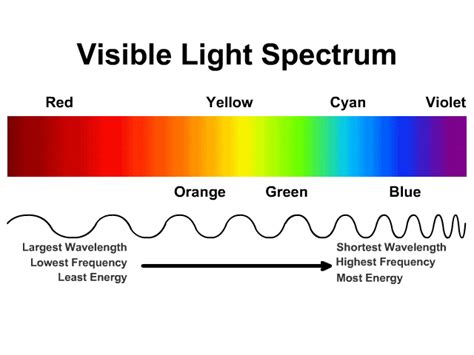
Table of Contents
What Color of Light Has the Most Energy? Unveiling the Secrets of the Electromagnetic Spectrum
The question of which color of light possesses the most energy is a fascinating one, diving deep into the fundamental principles of physics and the nature of light itself. While it might seem intuitive to associate brighter colors with more energy, the reality is far more nuanced and involves understanding the relationship between light's wavelength, frequency, and energy. This article will explore this relationship in detail, demystifying the connection between color and energy within the electromagnetic spectrum.
Understanding the Nature of Light
Before diving into the specifics of color and energy, it's crucial to establish a foundational understanding of light's true nature. Light isn't just something we see; it's electromagnetic radiation, a form of energy that travels in waves. These waves oscillate with specific frequencies and have corresponding wavelengths. The wavelength is the distance between successive crests of the wave, while the frequency represents the number of wave cycles passing a given point per second.
Key takeaway: Light is energy traveling in waves, characterized by its frequency and wavelength.
The Electromagnetic Spectrum: A Rainbow of Energy
The electromagnetic spectrum encompasses a vast range of electromagnetic radiation, categorized by its wavelength and frequency. Visible light, the portion we can perceive with our eyes, represents only a tiny fraction of this spectrum. The spectrum extends from incredibly long radio waves to extremely short gamma rays, each with its own distinct energy level.
From Radio Waves to Gamma Rays: A Spectrum of Energy Levels
The relationship between wavelength, frequency, and energy is inversely proportional. This means that as the wavelength decreases (the waves get shorter), the frequency increases, and consequently, the energy increases. Conversely, longer wavelengths correspond to lower frequencies and lower energy levels.
- Radio waves: Possessing the longest wavelengths and lowest frequencies, they carry the least energy.
- Microwaves: Shorter wavelengths than radio waves, they carry more energy, sufficient to heat food.
- Infrared radiation: Even shorter wavelengths, with enough energy to produce heat we can feel.
- Visible light: The narrow band we perceive as colors, ranging from red (longest wavelength) to violet (shortest wavelength).
- Ultraviolet radiation: Shorter wavelengths than visible light, carrying more energy and capable of causing sunburns.
- X-rays: Much shorter wavelengths and higher frequencies than ultraviolet light, with significantly higher energy.
- Gamma rays: Possessing the shortest wavelengths and highest frequencies, they carry the most energy in the electromagnetic spectrum.
Key takeaway: The electromagnetic spectrum shows a clear trend: shorter wavelengths (higher frequencies) mean higher energy.
Visible Light and Energy: The Color Connection
Within the visible light portion of the spectrum, violet light has the shortest wavelength and highest frequency, therefore carrying the most energy among visible colors. Red light, on the other hand, has the longest wavelength and lowest frequency, thus carrying the least energy within the visible spectrum. The other colors, such as orange, yellow, green, blue, and indigo, fall somewhere in between, with their energy levels directly correlated to their wavelengths and frequencies.
The Energy Hierarchy of Visible Light Colors
Here's a breakdown of the visible light spectrum in order of decreasing energy:
- Violet: Highest energy
- Indigo: High energy
- Blue: Moderate to high energy
- Green: Moderate energy
- Yellow: Moderate to low energy
- Orange: Low energy
- Red: Lowest energy
Key takeaway: Within visible light, violet light possesses the highest energy due to its shortest wavelength and highest frequency.
Practical Applications: Harnessing the Energy of Light
The differing energy levels of light have numerous practical applications across various scientific and technological fields. Here are just a few examples:
1. Photovoltaic Cells (Solar Panels)
Solar panels rely on the photovoltaic effect, where light energy is converted into electrical energy. Different colors of light contribute differently to this process. While they are designed to capture a broad range of the spectrum, those designed for higher efficiency might prioritize the higher-energy components like blue and violet light to maximize energy conversion.
2. Photography and Imaging
Different colors of light interact differently with photographic sensors or film. Understanding the energy levels of different colors is essential in optimizing image capture and processing.
3. Medical Treatments (Phototherapy)
Certain wavelengths of light are used in medical treatments, like photodynamic therapy for cancer treatment. The choice of wavelength depends on the targeted biological processes and the energy required to interact with specific molecules.
4. Laser Technology
Lasers emit light of a very specific wavelength and frequency. The choice of laser depends on the application – higher energy lasers (e.g., those emitting ultraviolet or blue light) are used for cutting and material processing, while lower energy lasers (e.g., red light) might be used for barcode scanning or laser pointers.
Beyond the Visible: The Significance of Higher-Energy Light
While violet light holds the title of highest energy within the visible spectrum, it's crucial to remember that far more energetic forms of light exist beyond our perception. Ultraviolet, X-rays, and gamma rays carry exponentially more energy than visible light. This higher energy translates to greater potential for both beneficial and harmful effects:
- Beneficial effects: X-rays are used in medical imaging, while ultraviolet light (in controlled amounts) helps our bodies produce Vitamin D.
- Harmful effects: Excessive exposure to UV radiation can cause sunburns and skin cancer; X-rays and gamma rays are highly ionizing and can damage living tissue.
Conclusion: The Energy Spectrum and its Implications
The relationship between color and energy is a complex but essential concept in understanding the nature of light. While violet light possesses the most energy within the visible spectrum, it's only a small part of the vast electromagnetic spectrum. The entire spectrum, from radio waves to gamma rays, represents a continuum of energy levels, each with its own unique properties and applications, showcasing the remarkable diversity and power of light as a fundamental form of energy. Understanding this spectrum, from the lowest energy radio waves to the highest energy gamma rays, is fundamental to advancements in various fields, from renewable energy technologies to medical diagnostics and treatments. The energy carried by different wavelengths of light plays a crucial role in shaping our world and driving scientific breakthroughs. Continued research into the interactions of light and matter promises even more fascinating discoveries and innovative applications in the future.
Latest Posts
Latest Posts
-
2 2x 2 X 12 0
Mar 14, 2025
-
1 1 4 Divided By 2 In Cups
Mar 14, 2025
-
The Most Reactive Metals Are The
Mar 14, 2025
-
Lewis Dot Diagram For 2 Individual Ions For Na
Mar 14, 2025
-
How Many Ounces Equals 2 Pounds
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Color Of Light Has The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
