What Are The Sides Of Dna Ladder Made Of
listenit
Mar 24, 2025 · 5 min read
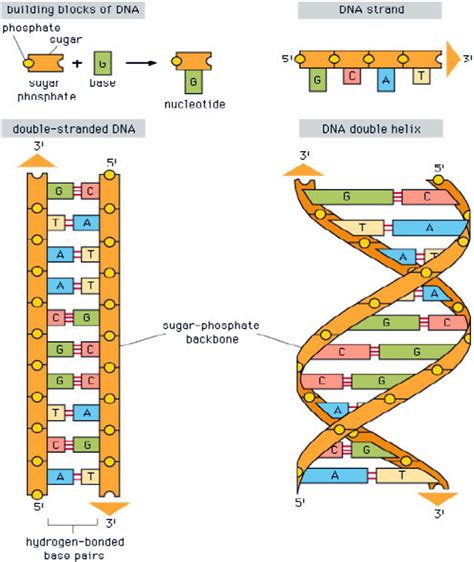
Table of Contents
What are the Sides of the DNA Ladder Made Of? Unraveling the Deoxyribose-Phosphate Backbone
The iconic double helix structure of DNA, often depicted as a twisted ladder, is a marvel of biological engineering. Understanding its components is crucial to grasping the mechanisms of heredity, gene expression, and numerous biological processes. This article delves deep into the composition of the DNA ladder's sides, exploring the deoxyribose-phosphate backbone and its crucial role in the molecule's stability and function.
The DNA Double Helix: A Closer Look
Before diving into the specifics of the sides, let's establish a foundational understanding of the DNA structure. DNA, or deoxyribonucleic acid, is a molecule composed of two polynucleotide chains that wind around each other to form a double helix. These chains are antiparallel, meaning they run in opposite directions. Think of it like a ladder twisted into a spiral staircase.
The "rungs" of this ladder are formed by pairs of nitrogenous bases: adenine (A) always pairs with thymine (T), and guanine (G) always pairs with cytosine (C). These base pairs are held together by relatively weak hydrogen bonds, allowing for easy separation during DNA replication and transcription.
But what about the sides, the backbone that holds these base pairs together? That's where the deoxyribose-phosphate backbone comes into play.
Deoxyribose: The Sugar Backbone
The sides of the DNA ladder are primarily composed of alternating deoxyribose sugar molecules and phosphate groups. Deoxyribose is a pentose sugar, meaning it contains five carbon atoms. It's a crucial component because its structure allows for the attachment of both the phosphate group and the nitrogenous base.
Key features of deoxyribose:
- Five-carbon structure: The five carbons are numbered 1' to 5', a crucial notation for understanding the linkage between the sugar and other components.
- Lack of a hydroxyl group: The key difference between deoxyribose and ribose (the sugar in RNA) is the absence of a hydroxyl group (-OH) at the 2' carbon. This seemingly small difference significantly affects the stability and structure of DNA, making it less reactive and more durable than RNA.
- Attachment points: The 3' carbon of one deoxyribose molecule links to the 5' carbon of the next via a phosphate group, creating the sugar-phosphate backbone. The 1' carbon is linked to the nitrogenous base.
Phosphate: Linking the Deoxyribose Units
Phosphate groups (PO₄³⁻) are negatively charged anions, and their presence is vital for several reasons:
- Backbone Linkage: They act as the bridge, linking the 3' carbon of one deoxyribose molecule to the 5' carbon of the next. This creates the continuous sugar-phosphate backbone that runs along each side of the DNA ladder. This phosphodiester bond is a strong covalent bond, ensuring the structural integrity of the DNA molecule.
- Negative Charge: The negative charges of the phosphate groups contribute to the overall negative charge of the DNA molecule. This charge is important for DNA interactions with proteins and other molecules within the cell. For instance, positively charged histone proteins interact with DNA to compact and organize it within chromosomes.
- Hydrophilic Nature: Phosphate groups are hydrophilic, meaning they are attracted to water. This property plays a role in DNA's interaction with the aqueous environment within the cell.
The 3' and 5' Ends: Directionality of the Backbone
The asymmetrical nature of the deoxyribose-phosphate backbone gives DNA its inherent directionality. One end of the strand has a free 3' hydroxyl group (-OH) on the deoxyribose sugar, while the other end has a free 5' phosphate group. This 3' to 5' directionality is crucial for DNA replication and transcription, as enzymes involved in these processes recognize and interact with the specific ends of the DNA molecule.
The Importance of the Deoxyribose-Phosphate Backbone
The deoxyribose-phosphate backbone isn't just a passive structural element; its properties are fundamental to DNA's function:
- Structural Stability: The strong covalent bonds between deoxyribose and phosphate contribute significantly to the overall stability of the DNA molecule, protecting the genetic information it carries. This stability is crucial for the long-term storage and transmission of genetic information across generations.
- Flexibility: Despite its structural rigidity, the backbone exhibits a degree of flexibility, allowing the DNA molecule to bend and twist, facilitating processes like DNA packaging within chromosomes and protein-DNA interactions.
- Accessibility of Bases: The arrangement of the backbone exposes the nitrogenous bases to the cellular environment, making them accessible for replication, transcription, and other molecular interactions.
- Regulation of Gene Expression: The backbone’s negative charge influences the interaction of DNA with various proteins involved in gene expression. These proteins often possess positively charged regions that bind to the DNA, influencing gene activation or repression.
Variations and Modifications to the Backbone
While the standard deoxyribose-phosphate backbone is the foundation of DNA structure, variations and modifications can occur:
- Methylation: The addition of a methyl group (CH₃) to certain bases or even the sugar backbone can alter gene expression. This epigenetic modification is a significant mechanism for regulating gene activity without altering the underlying DNA sequence.
- Glycosylation: Attachment of sugar molecules to the backbone can influence DNA stability and interactions with other molecules.
- Damage: The DNA backbone can be damaged by various factors, including radiation and certain chemicals. These damages can lead to mutations and potentially harmful consequences. Cellular repair mechanisms exist to address such damages and maintain genomic integrity.
Conclusion: A Masterpiece of Molecular Design
The sides of the DNA ladder, composed of the deoxyribose-phosphate backbone, are far more than simple structural elements. This seemingly simple repeating unit plays a multifaceted role in maintaining DNA's stability, ensuring the accessibility of genetic information, and participating in crucial cellular processes. Understanding the structure and function of the deoxyribose-phosphate backbone provides a deeper appreciation of the intricate workings of life at a molecular level, furthering our understanding of genetics, heredity, and numerous related biological fields. The backbone's unique properties, including its stability, directionality, and inherent negative charge, highlight the elegance and efficiency of the molecular design of life's blueprint. Future research into the intricacies of DNA backbone modification and its role in various biological processes promises to yield further insights into the complexity and beauty of this remarkable molecule. The ongoing exploration of DNA's structural features and functional roles remains a cornerstone of modern biological research.
Latest Posts
Latest Posts
-
How Many Radians Are In A Revolution
Mar 25, 2025
-
3 4 To The Power Of 2
Mar 25, 2025
-
What Is 0 125 As A Percent
Mar 25, 2025
-
2 Yards Is How Many Inches
Mar 25, 2025
-
Can Igneous Rock Become Sedimentary Rock
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Are The Sides Of Dna Ladder Made Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
