What Are Rows On The Periodic Table Called
listenit
Mar 16, 2025 · 6 min read
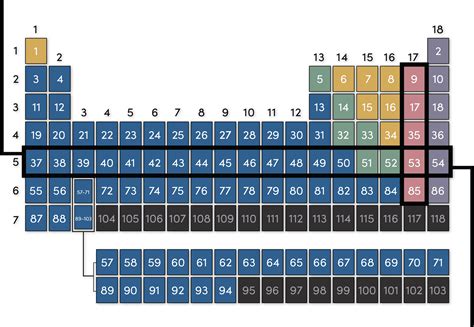
Table of Contents
What Are Rows on the Periodic Table Called? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes chemical elements in a structured manner, revealing patterns and relationships between them. While many are familiar with the columns, called groups or families, fewer understand the significance of the rows, which are known as periods. This article delves into the concept of periods on the periodic table, explaining what they represent, their properties, and their crucial role in understanding the behavior of elements.
Understanding Periods: A Horizontal Journey Through Electron Shells
Periods in the periodic table are the horizontal rows that organize elements based on their electron shell structure. Each period corresponds to a principal energy level or shell where electrons are located around an atom's nucleus. As you move across a period from left to right, you sequentially add electrons to the outermost shell, the valence shell. This progressive addition of electrons significantly impacts the chemical and physical properties of the elements within that period.
Period 1: The Simplest Beginnings
The first period, the shortest, consists of only two elements: hydrogen (H) and helium (He). These elements possess electrons only in the first principal energy level (n=1), which can hold a maximum of two electrons. Hydrogen, with one electron, is highly reactive, while helium, with a full outermost shell, is an inert noble gas. This stark contrast illustrates the fundamental influence of electron configuration on an element's properties.
Period 2: Introducing the s and p Blocks
Period 2 introduces a more complex scenario. It includes eight elements, starting with lithium (Li) and ending with neon (Ne). These elements fill both the s and p subshells within the second principal energy level (n=2). This period showcases the increasing electronegativity and decreasing metallic character as we move from left to right. Lithium and beryllium are reactive metals, while boron, carbon, nitrogen, oxygen, and fluorine are nonmetals with varying degrees of reactivity. Neon, with a complete outermost shell, is another inert noble gas.
Period 3: Expanding the s and p Blocks
Period 3 mirrors the pattern established in period 2, also featuring eight elements, from sodium (Na) to argon (Ar). Similar trends in electronegativity and metallic character are observed, emphasizing the influence of the valence electron configuration. The elements in this period exhibit a wider range of physical properties and chemical reactivity.
Periods 4 and 5: Introducing the d Block - Transition Metals
Periods 4 and 5 mark a significant shift, introducing the d subshell. These longer periods, containing 18 elements each, include the transition metals. The transition metals are characterized by their variable oxidation states, which contribute to their diverse and often colorful compounds. The filling of the d orbitals leads to a less dramatic change in properties across the period compared to the s and p block elements.
Periods 6 and 7: The f Block - Inner Transition Metals and the Lanthanides & Actinides
Periods 6 and 7 are the longest, with 32 elements each. These periods incorporate the f subshell, resulting in the inclusion of the inner transition metals: the lanthanides (rare earth elements) in period 6 and the actinides in period 7. The f orbitals are deeply embedded within the atom, leading to subtle variations in chemical properties within these series. The actinides, in particular, are known for their radioactivity.
Properties Across a Period: Trends and Patterns
As we traverse a period from left to right, several key properties exhibit consistent trends:
-
Atomic Radius: Generally decreases. This is because, while an additional electron shell is added, the increasing nuclear charge pulls the electrons closer to the nucleus.
-
Electronegativity: Generally increases. Electronegativity measures an atom's ability to attract electrons in a chemical bond. As the nuclear charge increases across a period, the attraction for bonding electrons strengthens.
-
Ionization Energy: Generally increases. Ionization energy is the energy required to remove an electron from an atom. The stronger nuclear charge across a period makes it more difficult to remove an electron.
-
Metallic Character: Generally decreases. Metallic character refers to properties like conductivity, malleability, and ductility. These properties generally decrease as we move across a period from left (metals) to right (nonmetals).
-
Electron Affinity: Shows a less straightforward trend, exhibiting both increases and decreases across a period. Electron affinity represents the energy change when an atom gains an electron.
These periodic trends are crucial for predicting the reactivity and chemical behavior of elements. They provide a framework for understanding the formation of chemical bonds and the properties of compounds.
The Significance of Periods in Chemistry
The concept of periods is fundamental to understanding numerous aspects of chemistry:
-
Predicting Chemical Reactivity: By examining the position of an element within a period and knowing the trends of properties across periods, we can predict its reactivity with other elements.
-
Understanding Chemical Bonding: The electron configuration, determined by the period, dictates the type of chemical bonds an element can form (ionic, covalent, metallic).
-
Explaining Physical Properties: The periodic trends help us understand the physical properties of elements such as melting point, boiling point, density, and conductivity.
-
Developing New Materials: The understanding of periods is crucial in the design and development of new materials with specific properties.
-
Nuclear Chemistry: The placement of radioactive elements within periods provides insight into their decay processes and associated properties.
Beyond the Basics: Further Exploration of Periodic Trends
The trends discussed above are generalizations, and deviations can occur due to various factors, including electron-electron repulsions and the presence of filled and half-filled subshells. For instance, the electron affinity trend is not as straightforward as the ionization energy trend. Specific elements might exhibit anomalies due to their unique electron configurations.
Furthermore, the concept of effective nuclear charge – the net positive charge experienced by an electron – plays a crucial role in explaining the periodic trends. The effective nuclear charge is not simply the total number of protons, but rather the nuclear charge minus the shielding effect of the inner electrons. This nuanced understanding of effective nuclear charge enhances the predictive power of periodic trends.
Conclusion: Periods - The Foundation of Chemical Understanding
The rows on the periodic table, known as periods, are more than just a simple organizational tool. They represent a fundamental principle in chemistry, reflecting the systematic addition of electrons to successive energy levels. Understanding the concept of periods and the trends in properties across periods is vital for predicting chemical reactivity, explaining chemical bonding, and understanding the physical properties of elements. As we continue to explore the complexities of the chemical world, the significance of periods in the periodic table remains an indispensable cornerstone of our understanding. The periodic table itself, and the principles it embodies, offers a testament to the elegance and interconnectedness of the natural world. From the simplest elements to the most complex molecules, the periods provide a roadmap to navigating the vast landscape of chemical knowledge.
Latest Posts
Latest Posts
-
What Is 8 In Fraction Form
Mar 17, 2025
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Are Rows On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
