What Are Rows Called In The Periodic Table
listenit
Mar 14, 2025 · 6 min read
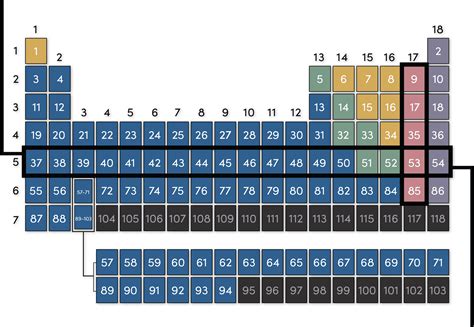
Table of Contents
What Are Rows Called in the Periodic Table? Understanding Periods and Their Significance
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While many are familiar with the table's columns, called groups or families, understanding the rows is equally crucial to grasping the fundamental principles of chemistry. So, what are rows called in the periodic table? They're called periods. This article delves deep into the meaning of periods, exploring their significance in predicting element properties, understanding electron configurations, and appreciating the overall organization of the periodic table.
Understanding Periodic Table Periods: A Detailed Explanation
Each row, or period, in the periodic table represents a principal energy level or shell within an atom. The elements within a period share the same highest principal quantum number (n), which dictates the energy level of their outermost electrons – the valence electrons. These valence electrons play a crucial role in determining an element's chemical behavior and reactivity. As we move across a period, from left to right, the number of protons and electrons increases, leading to systematic changes in atomic size, ionization energy, electronegativity, and other properties.
Period 1: The Simplest Period
The first period contains only two elements: hydrogen (H) and helium (He). Both elements have their electrons in the first energy level (n=1), which can hold a maximum of two electrons. This shell is closest to the nucleus, resulting in strong nuclear attraction and smaller atomic radii for these elements.
Period 2 and Period 3: The Beginning of Trends
Period 2 and Period 3 showcase the beginning of observable trends in properties. As we move across these periods, we progress through alkali metals (highly reactive), alkaline earth metals (less reactive than alkali metals), transition metals (with variable oxidation states), metalloids (with intermediate properties), and finally, nonmetals (like halogens and noble gases). These variations are directly related to the gradual filling of the second and third electron shells.
Periods 4 and 5: Introducing d-block Elements
Periods 4 and 5 are longer than the previous periods because they incorporate the transition metals. The transition metals are characterized by the filling of the d orbitals (d subshells), resulting in the characteristic properties like variable oxidation states, formation of coloured compounds, and catalytic activity.
Periods 6 and 7: The Lanthanides and Actinides
Periods 6 and 7 are even longer due to the inclusion of the f-block elements – the lanthanides and actinides, respectively. These elements are placed separately at the bottom of the table for aesthetic reasons; otherwise, the table would be excessively wide. The filling of the f orbitals contributes to the unique properties of these elements, many of which are radioactive.
The Significance of Periods in Predicting Element Properties
The periodic arrangement of elements within periods allows us to predict certain properties based on an element's position. Several key properties exhibit systematic changes as you move across a period:
Atomic Radius: The Size of Atoms
Atomic radius generally decreases across a period. This trend is due to the increasing nuclear charge (number of protons) while adding electrons to the same principal energy level. The stronger attraction between the nucleus and electrons leads to a smaller atomic size.
Ionization Energy: Removing Electrons
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The increased nuclear charge holds the electrons more tightly, making it more difficult to remove them. Exceptions to this trend arise due to electron shielding and electron configuration effects.
Electronegativity: Attraction for Electrons
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also generally increases across a period. Elements on the right side of the periodic table, particularly the halogens, are highly electronegative due to their strong attraction for electrons.
Metallic Character: A Gradual Shift
Metallic character, the tendency to lose electrons and form positive ions, generally decreases across a period. Metals are located on the left side of the table, while nonmetals are on the right. Metalloids occupy the intermediate region, exhibiting properties of both metals and nonmetals.
Electron Configuration and the Periods
The period number directly corresponds to the principal quantum number (n) of the valence electrons. For example, elements in period 2 have their valence electrons in the n=2 energy level (2s and 2p orbitals), while elements in period 3 have valence electrons in the n=3 energy level (3s and 3p orbitals). Understanding this relationship is essential for predicting electron configurations and understanding chemical bonding. The systematic filling of orbitals across periods explains the recurring patterns of chemical properties.
Beyond the Basic Trends: Exceptions and Irregularities
While the periodic table provides a useful framework for predicting element properties, it’s crucial to acknowledge that exceptions exist. These exceptions often stem from subtle variations in electron-electron repulsions, shielding effects, and the stability of half-filled and fully-filled subshells (Hund's rule). These deviations highlight the complexity of atomic structure and the limitations of simplistic models.
The Importance of Periods in Chemical Reactions
The periodic trends within periods directly influence the reactivity and chemical behavior of elements. For instance, alkali metals in the first group (period 1 and beyond) readily lose one electron to achieve a stable noble gas configuration, making them highly reactive with other elements. Conversely, halogens in group 17 are highly reactive because they readily gain one electron to also achieve a stable noble gas configuration. Understanding these periodic trends is key to predicting the products and outcomes of various chemical reactions.
Practical Applications and Further Exploration
The understanding of periods is crucial in various fields:
- Predicting chemical reactions: Knowledge of periodic trends allows chemists to anticipate the reactivity and the type of chemical bonds formed between elements.
- Material Science: The properties of elements within a given period influence the characteristics of materials they form. For instance, the period affects the conductivity, strength, and other physical properties of alloys and other materials.
- Nuclear Chemistry: The arrangement of elements within periods is particularly significant in nuclear chemistry, especially when dealing with radioactive elements found in the later periods.
- Analytical Chemistry: Understanding the periodic table helps analytical chemists identify and quantify elements in various samples.
The periodic table’s organization by periods is not just a convenient arrangement; it reflects the fundamental laws of physics and chemistry that govern the behavior of matter. Further exploration into quantum mechanics and atomic structure will reveal the deeper reasons behind these periodic trends. The importance of periods goes beyond simple memorization; it's the key to understanding the intricate relationships between elements and their remarkable properties.
Conclusion: Periods – The Foundation of Chemical Understanding
In summary, the rows in the periodic table are called periods. They represent principal energy levels, and understanding their significance is vital to grasping the fundamental principles of chemistry. The systematic variations in properties across a period, including atomic radius, ionization energy, electronegativity, and metallic character, are all directly linked to the filling of electron orbitals within that specific energy level. The concepts discussed here form a foundation for a deeper understanding of chemical bonding, reactivity, and material science. Mastering the principles of periods and their relation to the properties of elements is essential for any serious student or professional involved in chemistry or any related scientific discipline. The periodic table, with its arrangement of elements into periods and groups, remains a powerful tool for organizing and understanding the vast array of chemical elements that make up our world.
Latest Posts
Latest Posts
-
X Square Root Of X 6
Mar 14, 2025
-
What Is The Square Root Of 33
Mar 14, 2025
-
What Is The Square Root Of 136
Mar 14, 2025
-
How To Find Z Score Without Standard Deviation
Mar 14, 2025
-
What Is 30 Percent Of 400
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about What Are Rows Called In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
