Water Boiling Physical Or Chemical Change
listenit
Mar 16, 2025 · 5 min read
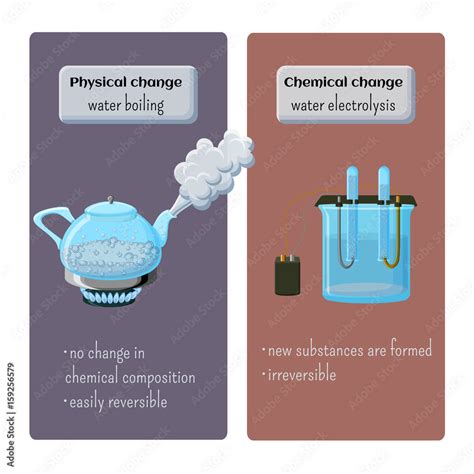
Table of Contents
Is Boiling Water a Physical or Chemical Change? A Deep Dive
The simple act of boiling water seems straightforward, but the underlying science reveals a fascinating interplay between physics and chemistry. Is it a physical change, a chemical change, or a blend of both? This in-depth exploration will unravel the mysteries behind boiling water, examining the processes involved, debunking common misconceptions, and offering a comprehensive understanding of this fundamental phenomenon.
Understanding the Nature of Physical and Chemical Changes
Before diving into the specifics of boiling water, let's establish a clear understanding of what constitutes a physical change versus a chemical change.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. Think about cutting paper, melting ice, or dissolving sugar in water. These actions change the physical state or form of the substance but don't create a new substance with different properties. The original substance can be recovered through a simple physical process (like freezing the melted ice).
Chemical changes, on the other hand, involve the transformation of one or more substances into entirely new substances with different chemical properties. Burning wood, rusting iron, and baking a cake are examples of chemical changes. The original substances are fundamentally altered, forming new compounds with distinct characteristics. The original substances cannot be easily recovered through simple physical means.
Boiling Water: A Primarily Physical Transformation
Boiling water is predominantly a physical change. The process involves a phase transition from liquid water to gaseous water vapor (steam), but the chemical composition remains unchanged. Water molecules (H₂O) in their liquid form simply gain enough kinetic energy to overcome the intermolecular forces holding them together, transitioning into a gaseous state.
The Role of Heat Energy
Heat energy is the driving force behind boiling. As you apply heat to water, the molecules absorb this energy, increasing their vibrational and translational motion. This increased kinetic energy weakens the hydrogen bonds between water molecules, allowing them to move more freely.
The Boiling Point and Phase Transition
Water's boiling point at standard atmospheric pressure (1 atm) is 100°C (212°F). At this temperature, the vapor pressure of water equals the atmospheric pressure, allowing bubbles of water vapor to form and escape from the liquid. This is the characteristic bubbling you observe when water boils. The temperature remains constant at 100°C (at 1 atm) until all the liquid water has turned into steam.
Recovering the Original Substance
Crucially, the steam produced during boiling can be condensed back into liquid water simply by cooling it. This demonstrates that no new chemical substance has been created; only the physical state has changed. This reversibility is a hallmark of a physical change.
Debunking Common Misconceptions
Several misconceptions surround the nature of boiling water. Let's address some of them:
Misconception 1: Boiling water changes chemically because it produces steam.
Reality: The steam is simply water in a gaseous state. The chemical formula (H₂O) remains unchanged. It's a phase transition, not a chemical transformation.
Misconception 2: Minerals in water change chemically during boiling.
Reality: While some minerals may precipitate out of solution (form solids) as the water evaporates, this is still primarily a physical change. The chemical composition of the minerals themselves generally remains unaltered. This is a process of concentration, not a chemical reaction. The minerals were already present in the water; boiling simply changes their state (from dissolved to solid).
Misconception 3: Boiling water causes chemical changes in substances dissolved in it.
Reality: While boiling might affect the concentration of dissolved substances (like salts), it rarely alters their chemical composition unless the temperature is high enough to trigger a chemical reaction between the dissolved substance and water or with itself. At typical boiling temperatures, this is not usually the case.
The Subtle Chemical Aspects: Dissociation and Decomposition
Although boiling water is primarily a physical change, there are some subtle chemical aspects to consider:
-
Dissociation of Water Molecules: A tiny fraction of water molecules undergo dissociation into hydronium ions (H₃O⁺) and hydroxide ions (OH⁻). This is an equilibrium process, meaning that the rate of formation of ions equals the rate of their recombination. Boiling does not significantly alter the equilibrium constant for this reaction, but it can slightly increase the concentration of ions because the increased temperature slightly enhances the dissociation. However, this is a negligible change in the overall chemical composition.
-
Decomposition at Extremely High Temperatures: At extremely high temperatures (significantly above 100°C), water can begin to decompose into hydrogen and oxygen gases. This is a chemical change (2H₂O → 2H₂ + O₂). However, this only occurs at temperatures far beyond those encountered during typical boiling.
Practical Applications and Implications
Understanding the nature of boiling water has numerous practical implications:
-
Cooking: Boiling is used extensively in cooking to soften foods, sterilize utensils, and extract flavors. The process is primarily physical but influences the chemical properties of food through things like denaturation of proteins.
-
Water Purification: Boiling water is a common method for eliminating harmful microorganisms. This is due to the heat killing the microbes, a primarily physical effect.
-
Steam Generation: Boiling water is crucial in generating steam for power plants, industrial processes, and heating systems. The steam's properties are directly linked to the physical change of water boiling.
-
Scientific Experiments: Boiling is used in many scientific experiments that involve separating mixtures, creating solutions and determining physical constants.
Conclusion: Boiling Water - A Physical Change with Subtle Chemical Nuances
In conclusion, while some subtle chemical processes might accompany boiling water, it is primarily a physical change. The transformation from liquid to gaseous water involves a change in physical state but does not alter the chemical composition of the water molecules (H₂O). This understanding is crucial for various applications in cooking, industrial processes, and scientific research. The apparent simplicity of boiling water belies the intricate interplay of physics and chemistry involved in this fundamental process. Remembering the distinctions between physical and chemical changes offers a key to understanding many other natural phenomena.
Latest Posts
Latest Posts
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Water Boiling Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
