Titration Of A Strong Base With A Strong Acid
listenit
Mar 12, 2025 · 7 min read
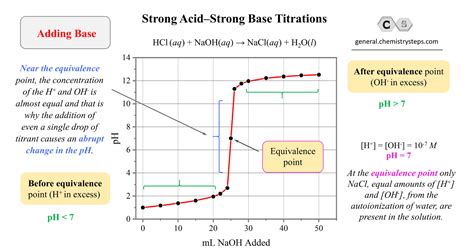
Table of Contents
Titration of a Strong Base with a Strong Acid: A Comprehensive Guide
Titration is a fundamental analytical technique in chemistry used to determine the concentration of an unknown solution, known as the analyte, by reacting it with a solution of known concentration, called the titrant. This article delves into the specifics of titrating a strong base with a strong acid, exploring the underlying chemistry, the calculations involved, and practical considerations for conducting accurate and reliable titrations.
Understanding Strong Acids and Strong Bases
Before diving into the titration process, it's crucial to understand the properties of strong acids and strong bases. Strong acids completely dissociate in water, releasing all their protons (H⁺ ions). Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃), and perchloric acid (HClO₄). Strong bases completely dissociate in water, releasing hydroxide ions (OH⁻ ions). Common examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and lithium hydroxide (LiOH).
The complete dissociation is key to understanding the titration curve. Because the reaction goes to completion, we can accurately predict the pH at various points during the titration.
The Neutralization Reaction
The reaction between a strong acid and a strong base is a neutralization reaction, producing water and a salt. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
This reaction is essentially a proton transfer from the acid to the base, forming water. The salt formed, in this case, sodium chloride (NaCl), is a neutral salt, meaning it doesn't significantly affect the pH of the solution.
The Titration Process: Step-by-Step
The titration of a strong base with a strong acid involves carefully adding the acid to the base until the equivalence point is reached. This process requires precise measurements and careful observation.
1. Preparation:
- Prepare the solution of the strong base: The concentration of the base should be known or determined beforehand through a standardization process.
- Prepare the standard solution of the strong acid: The concentration of the acid must be accurately known. This is often achieved using a primary standard, a highly pure substance that can be weighed accurately.
- Clean and rinse the glassware: Burets, pipettes, and flasks must be thoroughly cleaned and rinsed with distilled water to avoid contamination.
- Prepare the indicator: An appropriate indicator, such as phenolphthalein, is chosen. Phenolphthalein is colorless in acidic solutions and pink in basic solutions. The color change of the indicator signals the endpoint of the titration.
2. Performing the Titration:
- Transfer a known volume of the strong base: Use a pipette to accurately transfer a known volume of the base solution into a clean Erlenmeyer flask.
- Add the indicator: Add a few drops of the chosen indicator to the base solution.
- Fill the buret with the strong acid: Fill a buret with the standard strong acid solution, ensuring no air bubbles are present. Record the initial buret reading.
- Titrate slowly: Slowly add the acid from the buret to the base solution, swirling the flask constantly to ensure thorough mixing.
- Observe the color change: As the acid is added, the color of the solution will gradually change. Continue adding the acid dropwise near the endpoint, where the color change occurs rapidly.
- Record the final buret reading: Once the color change is complete and persistent, stop adding acid and record the final buret reading.
3. Calculations:
The volume of acid used to reach the equivalence point (the point where the moles of acid equal the moles of base) is used to calculate the concentration of the unknown base. The calculation utilizes the following formula:
MₐVₐ = MբVբ
Where:
- Mₐ = Molarity of the strong acid
- Vₐ = Volume of the strong acid used (in liters)
- Mբ = Molarity of the strong base (unknown)
- Vբ = Volume of the strong base used (in liters)
By rearranging the formula, we can calculate the molarity of the strong base (Mբ):
Mբ = (MₐVₐ) / Vբ
The Titration Curve
A titration curve is a graph of the pH of the solution versus the volume of titrant added. For the titration of a strong base with a strong acid, the curve has a characteristic shape. Initially, the pH is high (basic). As the acid is added, the pH decreases gradually. Near the equivalence point, the pH drops sharply, and then levels off at a low pH (acidic) after the equivalence point.
The Equivalence Point and the Endpoint
The equivalence point is the theoretical point at which the moles of acid added equal the moles of base initially present. The endpoint is the point at which the indicator changes color. Ideally, the endpoint and equivalence point should coincide, but a small difference may exist due to the indicator's limitations.
pH Calculations at Different Stages
Calculating the pH at different stages of the titration requires understanding the different chemical species present.
- Before the equivalence point: The pH is determined by the excess hydroxide ions from the strong base.
- At the equivalence point: The pH is exactly 7 because the strong acid and strong base completely neutralize each other, resulting in only water and a neutral salt.
- After the equivalence point: The pH is determined by the excess hydrogen ions from the strong acid.
Choosing the Right Indicator
The choice of indicator is crucial for obtaining accurate results. The indicator should change color near the equivalence point of the titration. Phenolphthalein is a commonly used indicator for strong acid-strong base titrations because its color change occurs around pH 8.3 – 10.0, which is close to the equivalence point of such titrations. Other indicators, such as methyl orange, might also be appropriate, depending on the specific titration and desired accuracy.
Sources of Error and How to Minimize Them
Several factors can introduce errors into titration experiments.
- Improper cleaning of glassware: Residues from previous experiments can affect the accuracy of measurements. Thorough cleaning and rinsing are crucial.
- Parallax error: Incorrect reading of the buret meniscus can lead to errors in volume measurement. Ensure the eye is at the same level as the meniscus when reading.
- Incorrect indicator choice: An indicator with a color change far from the equivalence point can lead to inaccurate results.
- Air bubbles in the buret: Air bubbles in the buret will cause errors in volume measurement. Ensure all air bubbles are removed before starting the titration.
- Improper mixing: Incomplete mixing of the solution during titration can lead to an inaccurate endpoint. Continuously swirl the flask to ensure good mixing.
- Impurities in the solutions: Impurities in the titrant or analyte can affect the accuracy of the results. Use high-purity chemicals whenever possible.
Applications of Strong Acid-Strong Base Titrations
Strong acid-strong base titrations are widely used in various fields, including:
- Determining the concentration of unknown solutions: This is the primary application, allowing for the precise determination of the concentration of acids or bases in various samples.
- Acid-base analysis in environmental monitoring: Determining the acidity or alkalinity of water samples is crucial in environmental monitoring and assessing water quality.
- Industrial quality control: Titration is essential in industrial settings to ensure the quality and consistency of manufactured products.
- Food and beverage analysis: Titration techniques are used to determine the acidity or alkalinity of foods and beverages.
- Pharmaceutical analysis: Purity and concentration analysis of pharmaceutical drugs often requires acid-base titration.
Conclusion
Titration of a strong base with a strong acid is a fundamental technique in analytical chemistry, offering a precise method for determining the concentration of an unknown solution. Mastering this technique requires a thorough understanding of the underlying chemistry, careful execution, and meticulous attention to detail. By following the steps outlined in this article and minimizing potential sources of error, accurate and reliable results can be obtained. The applications of this technique extend across various scientific and industrial fields, making it an indispensable tool in analytical laboratories worldwide. Understanding the titration curve and the calculations involved empowers chemists to accurately determine concentrations and further their analytical work. This comprehensive guide aims to provide a robust foundation for understanding and executing this crucial analytical method.
Latest Posts
Latest Posts
-
Can The Great Gatsby Be Seen As Satire
Mar 12, 2025
-
How Many Pints In 5 Quarts
Mar 12, 2025
-
How Many Valence Electrons Do Alkali Metals Have
Mar 12, 2025
-
What Is The Charge Of O2
Mar 12, 2025
-
What Two Elements Make Up Water
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Titration Of A Strong Base With A Strong Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
