This Type Of Reaction Requires Energy In Order To Proceed
listenit
Mar 15, 2025 · 6 min read
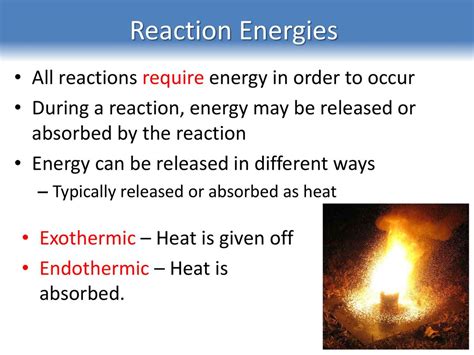
Table of Contents
Endothermic Reactions: When Energy Input Fuels Chemical Change
Endothermic reactions are a fascinating class of chemical processes that require an input of energy to proceed. Unlike exothermic reactions, which release energy in the form of heat or light, endothermic reactions absorb energy from their surroundings. This energy absorption can manifest in various ways, leading to a decrease in the temperature of the reaction system. Understanding endothermic reactions is crucial in various fields, from chemistry and biology to industrial processes and environmental science. This article delves deep into the nature of these reactions, exploring their characteristics, examples, and practical applications.
Understanding the Energy Barrier: Activation Energy
At the heart of any chemical reaction lies the concept of activation energy (Ea). This is the minimum amount of energy required for the reactants to overcome the energy barrier and initiate the reaction. In exothermic reactions, the energy released during the reaction is greater than the activation energy, resulting in a net release of energy. However, in endothermic reactions, the energy absorbed is greater than the energy released, leading to a net energy absorption. This means that an external energy source is needed to provide the activation energy and allow the reaction to proceed.
Think of it like pushing a boulder uphill. You need to expend energy (activation energy) to get the boulder moving. Once it starts rolling, it might gain momentum (exothermic, energy released), or you might need to continue pushing to keep it moving (endothermic, energy required continuously). The endothermic reaction is the uphill struggle, continuously requiring input.
Visualizing Endothermic Reactions with Energy Diagrams
Energy diagrams provide a visual representation of the energy changes during a reaction. For an endothermic reaction, the energy of the products is higher than the energy of the reactants. The difference between the energy of the products and reactants represents the net energy absorbed during the reaction. This is clearly illustrated by the upward slope of the energy diagram.
(Insert a simple energy diagram here showing reactants at a lower energy level than products, with a clearly marked activation energy hump.)
Characteristics of Endothermic Reactions
Several key characteristics distinguish endothermic reactions from their exothermic counterparts:
-
Energy Absorption: The most defining feature is the absorption of energy from the surroundings. This energy can be in the form of heat, light, or electrical energy.
-
Temperature Decrease: Because the reaction absorbs heat from its surroundings, the temperature of the reaction system often decreases. You might observe a cooling effect in the reaction vessel.
-
Positive Enthalpy Change (ΔH): The enthalpy change (ΔH) is a measure of the heat absorbed or released during a reaction at constant pressure. For endothermic reactions, ΔH is always positive, indicating that heat is absorbed.
-
Non-Spontaneous Nature: Many (though not all) endothermic reactions are non-spontaneous, meaning they don't occur naturally without an external energy input. They require a continuous supply of energy to proceed.
Examples of Endothermic Reactions
Endothermic reactions are prevalent in various aspects of our lives, both naturally occurring and in engineered systems. Here are some notable examples:
1. Photosynthesis: The Engine of Life
Photosynthesis, the process by which plants convert light energy into chemical energy in the form of glucose, is a prime example of an endothermic reaction. Plants absorb sunlight, carbon dioxide, and water to produce glucose and oxygen. The energy from sunlight drives this reaction, making it highly endothermic.
6CO₂ + 6H₂O + Light Energy → C₆H₁₂O₆ + 6O₂
2. Melting Ice: A Phase Transition
Melting ice cubes is another simple illustration. Heat from the surroundings is absorbed to break the hydrogen bonds holding the water molecules together in the ice crystal structure. This phase change from solid to liquid is an endothermic process.
3. Cooking an Egg: Denaturation of Proteins
The cooking of an egg involves denaturation of proteins, an endothermic process. Heat energy breaks the weak bonds holding the protein molecules in their specific three-dimensional structures, causing them to unfold and solidify.
4. Electrolysis of Water: Splitting Water Molecules
Electrolysis uses electrical energy to decompose water into hydrogen and oxygen gases. This process requires a significant input of electrical energy, making it highly endothermic.
2H₂O + Electrical Energy → 2H₂ + O₂
5. Dissolving Ammonium Nitrate in Water: A Cooling Effect
Dissolving ammonium nitrate (NH₄NO₃) in water is a classic example of an endothermic reaction often used in instant cold packs. The dissolution process absorbs heat from the surroundings, resulting in a noticeable temperature drop.
Applications of Endothermic Reactions
The principles of endothermic reactions are exploited in numerous applications across various fields:
1. Refrigeration and Air Conditioning: Harnessing Endothermic Processes
Refrigeration and air conditioning systems utilize endothermic processes to cool spaces. Refrigerants absorb heat from the air, undergoing an endothermic phase change, and then release this heat elsewhere in the system.
2. Industrial Processes: Driving Chemical Reactions
Many industrial processes involve endothermic reactions. For example, the production of certain chemicals requires significant energy input to initiate and sustain the reaction.
3. Instant Cold Packs: Utilizing Dissolution Endothermicity
Instant cold packs commonly used for injuries utilize the endothermic dissolution of ammonium nitrate in water to provide rapid cooling.
4. Scientific Research: Studying Reaction Kinetics and Thermodynamics
Endothermic reactions are studied extensively in scientific research to understand reaction kinetics, thermodynamics, and energy transfer mechanisms.
Factors Affecting Endothermic Reactions
Several factors can influence the rate and extent of an endothermic reaction:
-
Temperature: Increasing the temperature generally increases the rate of an endothermic reaction, providing more energy to overcome the activation energy barrier.
-
Concentration: Increasing the concentration of reactants often increases the rate of an endothermic reaction by increasing the frequency of collisions between reactant molecules.
-
Surface Area: Increasing the surface area of solid reactants increases the rate of an endothermic reaction by providing more contact points for the reaction to occur.
-
Catalyst: A catalyst can lower the activation energy of an endothermic reaction, increasing its rate without being consumed in the process.
Distinguishing Endothermic from Exothermic Reactions
It's crucial to differentiate between endothermic and exothermic reactions. While both involve energy changes, the direction of energy flow is opposite:
| Feature | Endothermic Reaction | Exothermic Reaction |
|---|---|---|
| Energy Flow | Absorbs energy from surroundings | Releases energy to surroundings |
| Temperature Change | Temperature decreases | Temperature increases |
| ΔH | Positive (ΔH > 0) | Negative (ΔH < 0) |
| Spontaneity | Often non-spontaneous | Often spontaneous |
| Examples | Photosynthesis, melting ice, dissolving NH₄NO₃ | Combustion, neutralization reactions, respiration |
Conclusion: The Significance of Endothermic Reactions
Endothermic reactions, though often requiring external energy input, play a vital role in numerous natural and industrial processes. Understanding their characteristics, applications, and the factors that influence them is crucial for advancements in various fields. From sustaining life through photosynthesis to developing efficient refrigeration systems, the principles of endothermic reactions are fundamental to our understanding of the world around us. Further research into optimizing these reactions and exploring their potential applications will continue to shape technological advancements and scientific discoveries.
Latest Posts
Latest Posts
-
1 2 X 1 2 Simplify
Mar 15, 2025
-
Do Plant Cells Have A Mitochondria
Mar 15, 2025
-
What Percent Of 40 Is 80
Mar 15, 2025
-
70 Of What Number Is 35
Mar 15, 2025
-
Words That Start With Same Letter
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about This Type Of Reaction Requires Energy In Order To Proceed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
