The Overall Charge Of The Nucleus Is
listenit
Mar 16, 2025 · 6 min read
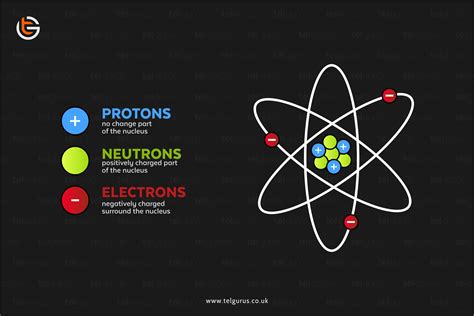
Table of Contents
The Overall Charge of the Nucleus: A Deep Dive into Atomic Structure and Properties
The overall charge of the nucleus is a fundamental concept in chemistry and physics, underpinning our understanding of atomic structure, chemical bonding, and the behavior of matter. This seemingly simple question opens a door to a fascinating world of subatomic particles, forces, and the intricate dance of protons and neutrons within the atom's core. This article will explore this concept in detail, examining its origins, implications, and the various factors that influence it.
Understanding the Nucleus: A Tiny Powerhouse
The atom, the basic building block of matter, is composed of three primary subatomic particles: protons, neutrons, and electrons. The nucleus, residing at the atom's center, is a densely packed region containing protons and neutrons. These particles, collectively known as nucleons, are bound together by the strong nuclear force, an incredibly powerful force that overcomes the electrostatic repulsion between the positively charged protons.
Protons: The Positive Charge Carriers
Protons are fundamental particles carrying a single positive charge (+1e), where 'e' represents the elementary charge (approximately 1.602 x 10⁻¹⁹ Coulombs). The number of protons in an atom's nucleus, known as the atomic number (Z), uniquely defines the element. For example, hydrogen (H) has one proton (Z=1), helium (He) has two (Z=2), and so on. This number is crucial because it determines the element's chemical properties and its place on the periodic table. The positive charge of the protons is the dominant factor in determining the overall charge of the nucleus.
Neutrons: The Neutral Partners
Neutrons, as their name suggests, carry no net electrical charge (0e). They contribute significantly to the nucleus's mass but play a more subtle role in its overall charge. The number of neutrons in an atom's nucleus can vary, leading to isotopes of the same element. Isotopes have the same number of protons but different numbers of neutrons, resulting in different mass numbers but identical chemical behavior.
The Strong Nuclear Force: Holding it All Together
The strong nuclear force is a fundamental force that binds protons and neutrons together within the nucleus, counteracting the electrostatic repulsion between the positively charged protons. This force is much stronger than the electromagnetic force at short distances, within the confines of the nucleus. Without the strong nuclear force, the nucleus would rapidly disintegrate due to the mutual repulsion of its protons.
Calculating the Overall Charge of the Nucleus
The overall charge of the nucleus is determined solely by the number of protons it contains. Since neutrons are electrically neutral, they do not contribute to the nucleus's net charge. Therefore, the calculation is straightforward:
Overall Charge of the Nucleus = (+1e) * Number of Protons = (+1e) * Z
Where:
- +1e represents the elementary positive charge of a proton.
- Z is the atomic number (number of protons).
For instance:
- Hydrogen (Z=1): Overall nuclear charge = (+1e) * 1 = +1e
- Helium (Z=2): Overall nuclear charge = (+1e) * 2 = +2e
- Carbon (Z=6): Overall nuclear charge = (+1e) * 6 = +6e
- Uranium (Z=92): Overall nuclear charge = (+1e) * 92 = +92e
This positive charge of the nucleus is crucial for the atom's overall neutrality and its interactions with other atoms.
The Role of the Nucleus in Atomic Stability and Chemical Reactions
The overall positive charge of the nucleus plays a pivotal role in determining the atom's stability and its interactions with other atoms.
Atomic Stability: The Balance of Forces
The stability of an atom's nucleus is a delicate balance between the strong nuclear force, which attracts protons and neutrons, and the electromagnetic force, which repels protons. For smaller atoms, the strong nuclear force easily overcomes the electrostatic repulsion. However, as the number of protons increases (higher atomic numbers), the electrostatic repulsion becomes increasingly significant, making the nucleus less stable. This is why very heavy elements are often radioactive, undergoing decay processes to achieve a more stable configuration.
Chemical Reactions and Bonding: The Influence of Charge
The positive charge of the nucleus directly impacts an atom's chemical behavior. The outermost electrons, weakly bound to the nucleus, are the primary participants in chemical reactions. The positive charge of the nucleus attracts these electrons, but the strength of this attraction decreases with increasing distance from the nucleus. This attraction governs how strongly an atom holds onto its electrons and how readily it can share or transfer them to form chemical bonds with other atoms. The arrangement of these electrons, dictated by the nuclear charge and quantum mechanical principles, determines an element's reactivity and its ability to form various chemical compounds.
Isotopes and Nuclear Properties: Variations on a Theme
As mentioned earlier, isotopes of the same element have the same number of protons (and thus the same nuclear charge) but differ in their number of neutrons. This difference affects the nucleus's mass and stability. Some isotopes are stable, while others are radioactive, undergoing decay processes such as alpha, beta, or gamma decay to reach a more stable configuration. These decay processes involve changes in the nucleus, altering the number of protons and neutrons, and ultimately, the element itself.
Beyond the Basics: Advanced Concepts
The overall charge of the nucleus is a fundamental concept that extends to more complex areas of physics and chemistry:
Nuclear Fission and Fusion: Harnessing Nuclear Energy
Nuclear fission and fusion processes involve manipulating the nucleus to release tremendous amounts of energy. In fission, a heavy nucleus splits into smaller nuclei, while in fusion, lighter nuclei combine to form a heavier nucleus. Both processes involve changes in the overall nuclear charge and release substantial energy, demonstrating the immense power locked within the atom's core.
Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI): Exploring the Nucleus's Magnetic Properties
While the overall charge of the nucleus is crucial, its magnetic properties, arising from the spin of protons and neutrons, are also important. Nuclear magnetic resonance (NMR) and its medical application, magnetic resonance imaging (MRI), utilize these magnetic properties to probe the structure and function of molecules and tissues. These techniques rely on the interaction of the nucleus's magnetic moment with an external magnetic field, providing valuable insights into various scientific fields.
Particle Physics: Delving into the Subatomic World
The study of the nucleus also leads us into the realm of particle physics. Protons and neutrons are themselves composed of smaller particles called quarks, held together by the strong force. Understanding the properties of quarks and the strong force is crucial to a complete understanding of the nucleus and its behavior.
Conclusion: The Significance of Nuclear Charge
The overall charge of the nucleus, determined by the number of protons, is a fundamental aspect of atomic structure and properties. This positive charge governs an atom's chemical behavior, its ability to form bonds, and its overall stability. Understanding the nucleus's charge is essential for comprehending a wide range of phenomena, from the formation of molecules to the release of nuclear energy. The concept extends to more advanced topics in chemistry, physics, and medicine, highlighting its central importance in our understanding of the world around us. Further exploration into the intricacies of nuclear physics and chemistry continues to reveal new insights into the remarkable power and complexity hidden within the atom's tiny core.
Latest Posts
Latest Posts
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
-
What Is 10 To The Power Of 7
Mar 17, 2025
-
Lowest Common Multiple Of 4 And 10
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about The Overall Charge Of The Nucleus Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
