Sides Of A Chemical Equation Called
listenit
Mar 26, 2025 · 5 min read
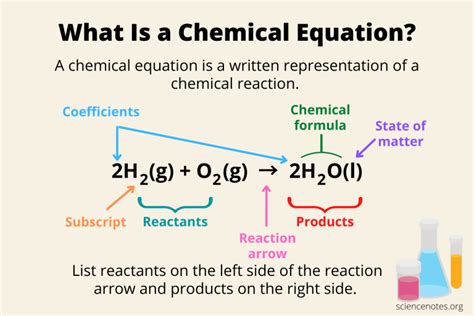
Table of Contents
Sides of a Chemical Equation: A Comprehensive Guide
Chemical equations are the shorthand language of chemistry, representing the transformation of reactants into products. Understanding the different sides of a chemical equation is crucial for grasping the fundamental principles of stoichiometry, balancing equations, and predicting reaction outcomes. This comprehensive guide delves into the intricacies of the reactant and product sides, exploring their significance and providing examples to solidify your understanding.
The Reactant Side: Where the Action Begins
The left-hand side of a chemical equation, often referred to as the reactant side, lists all the chemical formulas of the substances that are reacting or undergoing a change. These are the starting materials involved in the chemical process. Reactants can be elements (like hydrogen, H₂, or oxygen, O₂) or compounds (like water, H₂O, or sodium chloride, NaCl).
Key characteristics of the reactant side:
-
Representation: Reactants are represented by their chemical formulas, accurately reflecting the types and numbers of atoms involved. For example, in the equation for the combustion of methane (CH₄ + 2O₂ → CO₂ + 2H₂O), CH₄ and 2O₂ represent the methane and oxygen reactants, respectively.
-
State of Matter: Often, the physical state of each reactant is indicated using parentheses: (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water). This adds crucial information about the reaction conditions. For instance, 2Na(s) + Cl₂(g) → 2NaCl(s) clearly shows sodium as a solid, chlorine gas, and solid sodium chloride as the product.
-
Stoichiometric Coefficients: The numbers placed before the chemical formulas (e.g., the '2' in 2O₂) are called stoichiometric coefficients. They represent the relative number of moles of each reactant needed for the reaction to occur as written. These coefficients are vital for balancing the equation, ensuring the law of conservation of mass is obeyed.
-
Reactant Properties: The properties of the reactants—including their reactivity, concentration, and temperature—significantly influence the reaction rate and the overall outcome. A highly reactive reactant will generally lead to a faster reaction.
Examples of Reactant Sides
-
Formation of Water: 2H₂(g) + O₂(g) (Here, hydrogen gas and oxygen gas are the reactants)
-
Neutralization Reaction: HCl(aq) + NaOH(aq) (Hydrochloric acid and sodium hydroxide are the reactants)
-
Single Displacement Reaction: Zn(s) + 2HCl(aq) (Zinc and hydrochloric acid are the reactants)
-
Decomposition Reaction: 2H₂O₂(l) (Hydrogen peroxide is the reactant)
The Product Side: The Result of the Reaction
The right-hand side of a chemical equation is the product side, representing the chemical formulas of the newly formed substances resulting from the chemical reaction. These are the products of the reaction, and their properties differ from the reactants.
Key characteristics of the product side:
-
Formation: Products are formed due to the rearrangement or combination of atoms from the reactants. This rearrangement involves breaking and forming chemical bonds.
-
Chemical Formulas: Just like reactants, products are represented by their respective chemical formulas. These formulas detail the elements and the number of atoms present in each product molecule.
-
Stoichiometric Coefficients: Product molecules are also accompanied by stoichiometric coefficients that indicate their relative amounts formed in the reaction. These coefficients must be balanced with those on the reactant side.
-
Product Properties: Understanding the properties of products is essential for predicting the outcome of a reaction and designing appropriate separation techniques.
Examples of Product Sides
-
Formation of Water: 2H₂O(l) (Water is the product)
-
Neutralization Reaction: NaCl(aq) + H₂O(l) (Sodium chloride and water are the products)
-
Single Displacement Reaction: ZnCl₂(aq) + H₂(g) (Zinc chloride and hydrogen gas are the products)
-
Decomposition Reaction: 2H₂O(l) + O₂(g) (Water and oxygen gas are the products)
The Importance of Balancing Chemical Equations
The key principle underlying chemical equations is the law of conservation of mass, which states that mass cannot be created or destroyed in a chemical reaction. Therefore, the total mass of the reactants must equal the total mass of the products. This is achieved by balancing the chemical equation.
Balancing Techniques:
-
Balancing by Inspection: This method involves adjusting the stoichiometric coefficients until the number of atoms of each element is the same on both sides of the equation.
-
Algebraic Method: This more systematic approach involves assigning variables to the coefficients and solving a system of algebraic equations to find the balanced coefficients.
-
Redox Balancing: For redox reactions (involving electron transfer), more specialized balancing techniques are necessary, often using half-reactions.
Example of Balancing a Chemical Equation
Let's balance the equation for the combustion of propane (C₃H₈):
Unbalanced: C₃H₈ + O₂ → CO₂ + H₂O
Balanced: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
In this balanced equation, we have 3 carbon atoms, 8 hydrogen atoms, and 10 oxygen atoms on both the reactant and product sides.
Beyond the Basics: Advanced Concepts
Understanding the reactant and product sides extends beyond simple balancing. It allows us to delve into more complex aspects of chemical reactions:
1. Limiting Reactants and Excess Reactants
In many reactions, one reactant is completely consumed before the others. This reactant is the limiting reactant, as it limits the amount of product formed. The remaining reactants are called excess reactants. Identifying the limiting reactant is crucial for optimizing reaction yields.
2. Stoichiometric Calculations
Stoichiometry involves using the coefficients in a balanced chemical equation to calculate the quantities of reactants and products involved in a reaction. This includes calculating theoretical yields, percent yields, and limiting reactant quantities.
3. Reaction Kinetics and Equilibrium
The rates at which reactants are transformed into products are described by reaction kinetics. In reversible reactions, the concept of equilibrium describes the point where the rates of the forward and reverse reactions are equal. The relative amounts of reactants and products at equilibrium are crucial for understanding the reaction’s extent.
4. Reaction Mechanisms
Reaction mechanisms describe the step-by-step sequence of elementary reactions that constitute an overall reaction. These mechanisms provide insights into how reactants are transformed into products at a molecular level.
Conclusion: Mastering the Chemical Equation
The reactant and product sides of a chemical equation are not just symbols; they represent the fundamental transformation of matter. A thorough understanding of these sides, including balancing equations and performing stoichiometric calculations, is critical for mastering chemistry. By learning to interpret and manipulate these sides, you'll unlock a deeper comprehension of chemical reactions and their impact on our world. The principles explored here lay the foundation for further explorations in more advanced areas of chemistry, enabling you to analyze and predict chemical processes with confidence.
Latest Posts
Latest Posts
-
At What Point During Mitosis Has The Nuclear Membrane Reformed
Mar 29, 2025
-
Solve X 1 X 2 0
Mar 29, 2025
-
What Does Mean At The End Of A Sentence
Mar 29, 2025
-
What Is 11 20 As A Decimal
Mar 29, 2025
-
How Far Away Is Pluto In Light Years
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Sides Of A Chemical Equation Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
