Pxidation Number Of H In H20
listenit
Mar 14, 2025 · 5 min read
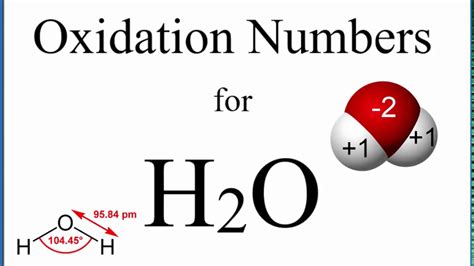
Table of Contents
Oxidation Number of H in H₂O: A Deep Dive
The seemingly simple question of the oxidation number of hydrogen in water (H₂O) belies a deeper understanding of fundamental chemical concepts. While many might immediately answer "+1," a nuanced exploration reveals the subtleties involved and the broader implications for understanding oxidation states in various chemical contexts. This comprehensive guide delves into the intricacies of determining the oxidation number of hydrogen in H₂O, exploring its exceptions and applications.
Understanding Oxidation Numbers
Before diving into the specific case of H₂O, let's establish a solid foundation on oxidation numbers. The oxidation number, also known as the oxidation state, is a number assigned to an atom in a molecule or ion that represents its apparent charge. It's a crucial concept in chemistry, particularly in:
- Redox reactions: Understanding oxidation numbers helps identify which species are being oxidized (losing electrons) and reduced (gaining electrons).
- Nomenclature: Oxidation numbers play a critical role in systematically naming compounds.
- Predicting reactivity: The oxidation state of an atom can offer insights into its potential to participate in chemical reactions.
Determining oxidation numbers involves a set of rules, often hierarchical, that must be applied systematically:
- Free elements: The oxidation number of an atom in its elemental form is always 0 (e.g., O₂ has an oxidation number of 0 for each oxygen atom).
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge (e.g., Na⁺ has an oxidation number of +1).
- Fluorine: Fluorine, the most electronegative element, always has an oxidation number of -1 in its compounds.
- Oxygen: Oxygen usually has an oxidation number of -2 in its compounds, except in peroxides (e.g., H₂O₂) where it's -1, and in compounds with fluorine where it's positive.
- Hydrogen: Hydrogen typically has an oxidation number of +1 in its compounds, except in metal hydrides (e.g., NaH) where it's -1.
- The sum of oxidation numbers: In a neutral molecule, the sum of the oxidation numbers of all atoms is zero. In a polyatomic ion, the sum of oxidation numbers equals the charge of the ion.
Determining the Oxidation Number of H in H₂O
Applying these rules to H₂O, we can readily determine the oxidation number of hydrogen. Oxygen, being more electronegative than hydrogen, typically has an oxidation number of -2. Since H₂O is a neutral molecule, the sum of the oxidation numbers must be zero. Let's represent the oxidation number of hydrogen as 'x':
2(x) + (-2) = 0
Solving for x:
2x = 2
x = +1
Therefore, the oxidation number of hydrogen in H₂O is +1.
Exceptions and Nuances
While the +1 oxidation state for hydrogen in H₂O is the most common and generally accepted, it's essential to acknowledge exceptions and nuances. The assignment of oxidation numbers is based on a model, and the actual charge distribution in a molecule can be more complex than a simple assignment of integer values would suggest.
One area where the oxidation number might appear less straightforward is in considering the electronegativity difference between oxygen and hydrogen. While oxygen is significantly more electronegative, the difference isn't absolute. The electron density is not entirely shifted to the oxygen atom; there's a degree of covalent character in the O-H bond. Advanced techniques, like computational chemistry methods, can provide more detailed insights into the actual charge distribution within the molecule, potentially revealing fractional oxidation states. However, for practical purposes and general chemical understanding, the +1 oxidation state remains a valid and useful approximation.
Hydrogen's Oxidation State in Other Compounds
It is important to note that the oxidation state of hydrogen is not always +1. As mentioned earlier, in metal hydrides like NaH, lithium hydride (LiH), and calcium hydride (CaH2), hydrogen exhibits an oxidation state of -1. In these compounds, hydrogen is more electronegative than the alkali or alkaline earth metal, causing it to gain an electron and achieve a -1 oxidation state. This highlights the dependence of the oxidation state on the chemical environment of the hydrogen atom.
Applications and Significance
Understanding the oxidation state of hydrogen, especially in water, has significant applications across various domains of chemistry:
- Redox reactions: In reactions involving water, the oxidation state of hydrogen (+1) can help identify whether it's being oxidized or reduced. For example, in the electrolysis of water, hydrogen ions are reduced to form hydrogen gas (H₂), where the oxidation state changes from +1 to 0.
- Balancing redox equations: Knowing the oxidation states of all species involved is critical for correctly balancing redox equations, ensuring that the number of electrons lost during oxidation equals the number of electrons gained during reduction.
- Predicting reaction pathways: The oxidation state can provide insights into the reactivity of different species and predict the direction of a reaction. For example, a species with a high oxidation state may be more likely to be reduced, while a species with a low oxidation state may be more likely to be oxidized.
- Understanding chemical bonding: While not directly representing the actual charge distribution, oxidation states provide a simplified model for understanding the distribution of electrons in a molecule and the types of chemical bonds involved.
Conclusion
The oxidation number of hydrogen in water is predominantly +1, a result derived from the application of established rules for assigning oxidation states. While the concept of oxidation numbers provides a simplified model, it remains a valuable tool for understanding chemical processes, predicting reaction pathways, and balancing redox reactions. It is crucial to acknowledge the limitations of this model and to understand the exceptions, like the -1 oxidation state in metal hydrides, to gain a comprehensive understanding of hydrogen's chemical behavior. The seemingly simple question of H's oxidation state in H₂O opens a door to a broader understanding of fundamental chemical principles and their practical applications. Further exploration of advanced chemical concepts will provide a more nuanced understanding of electron distribution and charge in molecules, refining our understanding of oxidation states beyond the simple integer assignments.
Latest Posts
Latest Posts
-
Ice Melting Physical Or Chemical Change
May 09, 2025
-
How Many 1 3 Cups To Make 2 3
May 09, 2025
-
How Did Cyanobacteria Affect Earths Early Atmosphere
May 09, 2025
-
Why Does Passive Transport Not Require Energy
May 09, 2025
-
Can Hydrogen Be A Central Atom
May 09, 2025
Related Post
Thank you for visiting our website which covers about Pxidation Number Of H In H20 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.