Process That Produces Ammonia Crossword Clue
listenit
Mar 16, 2025 · 6 min read
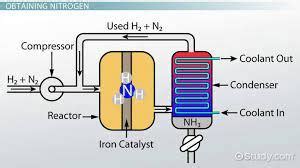
Table of Contents
The Haber-Bosch Process: Unraveling the Crossword Clue "Process That Produces Ammonia"
The crossword clue "Process that produces ammonia" almost certainly refers to the Haber-Bosch process. This industrial method is not only crucial for producing ammonia but also fundamentally important for sustaining our modern world's agricultural and industrial needs. Understanding its intricacies provides a fascinating insight into chemical engineering and its impact on society. Let's delve deep into the Haber-Bosch process, exploring its history, chemistry, industrial applications, and environmental considerations.
A Brief History: Revolutionizing Agriculture
Developed independently by Fritz Haber and Carl Bosch in the early 20th century, the Haber-Bosch process represents a pivotal moment in human history. Before its inception, the production of ammonia relied on limited natural sources, severely restricting the availability of nitrogen-based fertilizers. This constraint directly impacted agricultural output, limiting food production and hindering population growth. Haber's groundbreaking discovery of a catalytic method to synthesize ammonia from atmospheric nitrogen and hydrogen revolutionized agriculture, unlocking the potential for significantly increased crop yields. Bosch’s subsequent engineering work scaled the process to an industrial level, solidifying its impact on global food security. For their contributions, both Haber and Bosch were awarded Nobel Prizes – Haber in Chemistry (1918) and Bosch in Chemistry (1931).
However, it's crucial to acknowledge the controversial aspects of Haber's legacy. His expertise in chemical warfare during World War I tarnished his reputation, highlighting the dual-use nature of scientific discoveries and their potential for both beneficial and destructive applications.
The Chemistry Behind Ammonia Production: A Detailed Look
The core chemical reaction within the Haber-Bosch process is the synthesis of ammonia (NH₃) from nitrogen (N₂) and hydrogen (H₂):
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
This seemingly simple equation belies the complexities involved in achieving efficient and sustainable ammonia production. The reaction is exothermic (releases heat), but it's also characterized by several significant challenges:
- Reaction Kinetics: The reaction between nitrogen and hydrogen is inherently slow at ambient temperatures and pressures. This sluggishness necessitates the use of a catalyst to speed up the reaction rate.
- Equilibrium Limitations: The reaction is reversible, meaning that ammonia decomposes back into nitrogen and hydrogen under certain conditions. To maximize ammonia yield, the reaction requires high pressures and relatively low temperatures, which shifts the equilibrium towards the product side. However, these conditions must be balanced against the economic viability and technical feasibility of the process.
- Catalyst Selection: The Haber-Bosch process relies on a finely tuned catalyst, typically iron promoted with other components like potassium and aluminum oxides. The catalyst's function is to lower the activation energy of the reaction, enabling it to proceed at a practical rate. The catalyst's precise composition and preparation method significantly impact its efficiency and longevity.
The Industrial Process: High Pressure, High Stakes
The industrial implementation of the Haber-Bosch process involves several key stages:
-
Hydrogen Production: The primary source of hydrogen is natural gas (methane), through a process called steam reforming. Other sources include coal gasification and electrolysis of water. The production of hydrogen itself consumes significant energy and is a significant source of greenhouse gas emissions.
-
Nitrogen Production: Atmospheric nitrogen is used directly, as it is readily available and inexpensive. However, it requires purification to remove impurities that can poison the catalyst.
-
Purification: Both hydrogen and nitrogen are purified to remove contaminants such as carbon monoxide, sulfur compounds, and oxygen, which can deactivate the catalyst.
-
Compression: The purified gases are compressed to very high pressures, typically ranging from 150 to 350 atmospheres. This high pressure is crucial for shifting the equilibrium of the reaction towards ammonia production.
-
Catalysis: The compressed gas mixture is passed over a bed of the iron catalyst at temperatures ranging from 400 to 500°C. The catalyst facilitates the reaction between nitrogen and hydrogen, forming ammonia.
-
Ammonia Separation: The ammonia formed is separated from the unreacted nitrogen and hydrogen through liquefaction. Ammonia has a lower boiling point than nitrogen and hydrogen, allowing for efficient separation. The unreacted gases are recycled back into the reactor to maximize overall ammonia yield.
-
Product Storage and Distribution: The liquid ammonia is stored in pressurized tanks and transported for various applications.
Applications of Ammonia: Beyond Fertilizers
While ammonia's role in fertilizer production is paramount, its applications extend far beyond agriculture:
-
Fertilizers: Ammonia is the key building block for the production of various nitrogen-containing fertilizers, including urea, ammonium nitrate, and ammonium phosphate. These fertilizers are essential for boosting crop yields and meeting the global demand for food.
-
Industrial Chemicals: Ammonia serves as a crucial intermediate in the synthesis of numerous industrial chemicals, including nitric acid, nylon, and various plastics.
-
Refrigerant: Ammonia's excellent thermodynamic properties make it a viable refrigerant in industrial refrigeration systems. However, its toxicity necessitates careful handling and safety precautions.
-
Pharmaceuticals: Ammonia is used in the production of various pharmaceuticals and medicines.
-
Cleaning Products: Diluted ammonia solutions are utilized in household cleaning products due to their degreasing and disinfecting properties.
Environmental Considerations: A Double-Edged Sword
While the Haber-Bosch process has revolutionized food production and countless other industries, it also presents considerable environmental challenges:
-
Greenhouse Gas Emissions: The process is energy-intensive, with significant amounts of fossil fuels being consumed to produce hydrogen and drive the reaction. This contributes to greenhouse gas emissions, contributing to climate change.
-
Energy Consumption: The high pressures and temperatures required for the process demand substantial energy inputs, raising concerns about energy efficiency and sustainability.
-
Catalyst Production and Disposal: The manufacturing of catalysts and their subsequent disposal also pose environmental concerns, necessitating responsible practices to minimize their impact.
-
Ammonia Leakage and Emissions: Ammonia leakage from industrial facilities and during transportation can have detrimental effects on human health and the environment.
-
Eutrophication: Excessive use of nitrogen-based fertilizers can lead to eutrophication of water bodies, causing algal blooms that deplete oxygen and harm aquatic ecosystems.
Future Prospects: Towards a Greener Ammonia Production
The environmental impact of the Haber-Bosch process necessitates ongoing research and development to improve its sustainability. Several promising strategies are being explored:
-
Renewable Energy Sources: Shifting towards renewable energy sources, such as solar and wind power, for hydrogen production could significantly reduce the process's carbon footprint.
-
Electrochemical Ammonia Synthesis: Electrocatalytic methods for ammonia synthesis, powered by renewable electricity, offer a promising path toward a greener process.
-
Improved Catalysts: Research into more efficient and environmentally friendly catalysts could lower energy consumption and improve the overall process efficiency.
-
Process Optimization: Advanced process control and optimization techniques can help minimize energy consumption and reduce ammonia emissions.
-
Circular Economy Approaches: Implementing circular economy principles to recycle and reuse materials can lessen the environmental burden associated with catalyst production and disposal.
In conclusion, the Haber-Bosch process, the answer to the crossword clue "Process that produces ammonia," has profoundly shaped our world. Its impact on agriculture and industry is undeniable, yet its environmental consequences necessitate urgent efforts to enhance its sustainability. Continued research and innovation are crucial to ensure that this vital process continues to meet our needs while minimizing its adverse environmental impact, safeguarding both our present and future. The future of the Haber-Bosch process, and indeed our food security, hinges on addressing these challenges and embracing a more sustainable approach to ammonia production.
Latest Posts
Latest Posts
-
What Is The Lcm Of 2 And 8
Mar 16, 2025
-
How To Convert Rev Sec To Rad Sec
Mar 16, 2025
-
What Is 65 In Fraction Form
Mar 16, 2025
-
Which Of The Following Atoms Has The Largest Atomic Radius
Mar 16, 2025
-
What Is A Product Of Meiosis
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Process That Produces Ammonia Crossword Clue . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
