Number Of Valence Electrons In S
listenit
Mar 16, 2025 · 6 min read
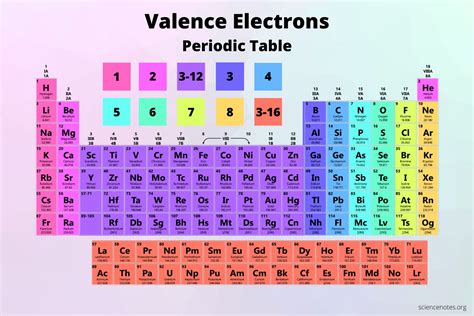
Table of Contents
The Number of Valence Electrons in the s-Block Elements: A Comprehensive Guide
Understanding valence electrons is crucial for comprehending chemical bonding and reactivity. This comprehensive guide delves into the number of valence electrons found in the elements of the s-block, explaining their significance and how this number dictates their chemical behavior. We'll explore the periodic table's organization, electron configurations, and the implications of valence electron counts for predicting chemical properties.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the most loosely held and, therefore, are most likely to participate in chemical bonding. They dictate how an atom will interact with other atoms, determining its reactivity and the types of bonds it can form (ionic, covalent, metallic). The number of valence electrons directly correlates to an element's position within the periodic table and its chemical properties.
The s-Block Elements: Location and Characteristics
The s-block elements are located in the first two columns (Groups 1 and 2) of the periodic table. They're characterized by their valence electrons occupying the s subshell. Group 1, the alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium), have one valence electron. Group 2, the alkaline earth metals (beryllium, magnesium, calcium, strontium, barium, and radium), have two valence electrons.
Group 1: Alkali Metals - One Valence Electron
Alkali metals are highly reactive due to their single valence electron. This lone electron is easily lost, resulting in the formation of a +1 cation. This readily gives them a strong tendency to participate in ionic bonding, forming stable ionic compounds with nonmetals. Their reactivity increases down the group, as the outermost electron becomes further from the nucleus and more easily lost. This explains why cesium and francium are amongst the most reactive elements.
- Example: Sodium (Na) has an electron configuration of 1s²2s²2p⁶3s¹. Its single valence electron in the 3s orbital is easily lost, forming the Na⁺ ion. This explains Sodium's high reactivity and its tendency to form ionic compounds like NaCl (sodium chloride).
Group 2: Alkaline Earth Metals - Two Valence Electrons
Alkaline earth metals have two valence electrons in their outermost s subshell. These two electrons are also relatively easy to lose, resulting in the formation of +2 cations. They are less reactive than alkali metals because losing two electrons requires more energy. Nevertheless, they still display significant reactivity, forming ionic compounds with nonmetals. Reactivity, like alkali metals, increases down the group.
- Example: Magnesium (Mg) has an electron configuration of 1s²2s²2p⁶3s². Its two valence electrons in the 3s orbital are readily lost to form the Mg²⁺ ion. This leads to its participation in ionic compounds like MgO (magnesium oxide).
Electron Configuration and Valence Electrons
Understanding electron configuration is essential for determining the number of valence electrons. Electron configuration describes the arrangement of electrons within an atom's energy levels and subshells. The Aufbau principle, Hund's rule, and the Pauli exclusion principle govern this arrangement.
The s subshell can hold a maximum of two electrons. Therefore, the s-block elements, having their valence electrons in the s subshell, will always have either one or two valence electrons, depending on their group. Let's illustrate this with examples:
- Lithium (Li): 1s²2s¹ - One valence electron (2s¹)
- Beryllium (Be): 1s²2s² - Two valence electrons (2s²)
- Sodium (Na): 1s²2s²2p⁶3s¹ - One valence electron (3s¹)
- Magnesium (Mg): 1s²2s²2p⁶3s² - Two valence electrons (3s²)
- Potassium (K): 1s²2s²2p⁶3s²3p⁶4s¹ - One valence electron (4s¹)
- Calcium (Ca): 1s²2s²2p⁶3s²3p⁶4s² - Two valence electrons (4s²)
Note: While the inner electrons (those not in the outermost shell) contribute to the atom's overall charge and properties, they do not participate directly in chemical bonding. Only valence electrons are directly involved in forming chemical bonds.
Importance of Valence Electrons in Chemical Bonding
The number of valence electrons is the primary factor determining the type and number of bonds an atom can form. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gas configuration (a full outer shell). This is the basis of the octet rule (though there are exceptions).
Ionic Bonding
Atoms with one or two valence electrons (like alkali and alkaline earth metals) often lose these electrons to achieve a stable configuration, forming positively charged ions (cations). They then electrostatically interact with negatively charged ions (anions) formed by nonmetals which gain electrons to complete their outer shells. This electrostatic attraction constitutes ionic bonding.
Covalent Bonding
Atoms with multiple valence electrons may share electrons with other atoms to achieve a stable configuration, forming covalent bonds. This is particularly common among nonmetals. While not directly related to the s-block elements' primary bonding behavior, understanding this contrast clarifies the unique reactivity of s-block elements.
Trends in Reactivity within the s-Block
Reactivity within the s-block exhibits clear trends:
- Increased reactivity down the group (for both alkali and alkaline earth metals): As you move down a group, the atomic radius increases, and the outermost electron is further from the nucleus and more easily lost. This leads to increased reactivity.
- Alkali metals are more reactive than alkaline earth metals: Losing one electron is easier than losing two, leading to greater reactivity in alkali metals.
- Exception in Beryllium: Beryllium, despite being an alkaline earth metal, is less reactive than expected due to its small atomic size and high ionization energy.
Applications of s-Block Elements and their Compounds
The s-block elements and their compounds have numerous applications across various industries:
- Sodium (Na): Used in streetlights (sodium-vapor lamps), in the production of sodium hydroxide (NaOH) which is used in numerous industrial processes.
- Potassium (K): Essential nutrient for plants and animals; used in fertilizers.
- Magnesium (Mg): Used in lightweight alloys, in fire-retardant materials, and in certain medicines.
- Calcium (Ca): Essential for bone health; used in construction materials (cement, plaster), and in the purification of metals.
These applications stem directly from the unique chemical properties dictated by their valence electron configurations.
Conclusion
The number of valence electrons in the s-block elements – one for Group 1 and two for Group 2 – is paramount to understanding their chemical behavior and reactivity. Their electron configurations directly influence their tendency to form ions, participate in chemical bonding, and consequently, their extensive applications in various fields. This understanding is fundamental to chemistry and crucial for predicting the properties and reactions of these important elements. Further exploration into the intricacies of electron configuration and chemical bonding will solidify your grasp on the fascinating world of chemical reactivity. This knowledge lays the foundation for understanding more complex chemical systems and phenomena.
Latest Posts
Latest Posts
-
Where Does Transcription Take Place In A Prokaryotic Cell
Mar 16, 2025
-
Is Water A Biotic Or Abiotic Factor
Mar 16, 2025
-
How Many Ounces Are In A Fifth
Mar 16, 2025
-
60 Kilometers To Miles Per Hour
Mar 16, 2025
-
How Many Moles Are In 9 8 Grams Of Calcium
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In S . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
