Number Of Valence Electrons In Cl
listenit
Mar 23, 2025 · 6 min read
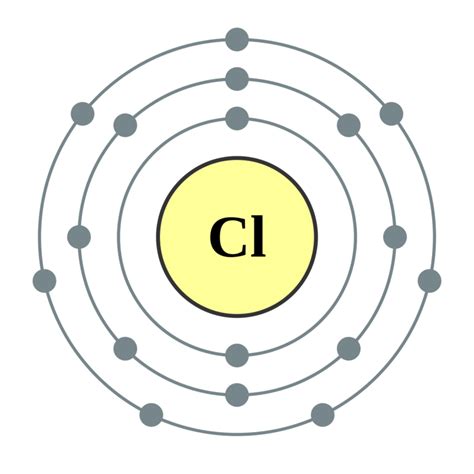
Table of Contents
Delving Deep into Chlorine's Valence Electrons: A Comprehensive Guide
Chlorine, a crucial element in our daily lives and a cornerstone of chemistry, holds a significant place in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its reactivity and bonding behavior. This article delves deep into the fascinating world of chlorine's valence electrons, exploring its electronic configuration, its implications for chemical bonding, and its role in various applications.
Understanding Valence Electrons: The Key to Reactivity
Before focusing specifically on chlorine, let's establish a solid foundation. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly dictates how an atom will interact with other atoms to achieve a stable electron configuration, usually resembling that of a noble gas. This stable configuration, often characterized by a full outermost shell, is the driving force behind chemical bonding.
Atoms strive to achieve this stability, either by gaining, losing, or sharing electrons. The method chosen heavily relies on the number of valence electrons. Atoms with nearly full valence shells tend to gain electrons, while those with only a few valence electrons tend to lose them. Atoms with approximately half-filled valence shells often share electrons, forming covalent bonds.
Chlorine's Electronic Configuration: Unraveling the Mystery
Chlorine (Cl), with an atomic number of 17, possesses 17 electrons. To determine the number of valence electrons, we need to understand its electronic configuration. Using the Aufbau principle and Hund's rule, we can arrange these electrons in various energy levels and sublevels.
The electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. This configuration tells us the distribution of electrons across different energy levels and orbitals. The numbers (1, 2, 3) represent the principal energy levels or shells, while the letters (s, p) represent the subshells, and the superscripts indicate the number of electrons in each subshell.
Breaking down the configuration further:
- 1s²: Two electrons occupy the first energy level's s subshell.
- 2s²: Two electrons occupy the second energy level's s subshell.
- 2p⁶: Six electrons occupy the second energy level's p subshell.
- 3s²: Two electrons occupy the third energy level's s subshell.
- 3p⁵: Five electrons occupy the third energy level's p subshell.
Crucially, the outermost shell (third energy level) contains seven electrons (two from the 3s subshell and five from the 3p subshell). This brings us to the key answer:
Chlorine has 7 valence electrons.
Implications of 7 Valence Electrons: Chemical Behavior of Chlorine
The presence of seven valence electrons significantly influences chlorine's chemical behavior. To attain a stable octet (eight electrons in its outermost shell) resembling the noble gas Argon, chlorine is highly likely to gain one electron. This electron gain leads to the formation of a chloride ion (Cl⁻), which carries a negative charge due to the extra electron.
This tendency to gain an electron makes chlorine a highly reactive element, particularly with metals. The reaction between chlorine and a metal involves the transfer of one electron from the metal atom to the chlorine atom, forming an ionic bond. This is exemplified in the formation of sodium chloride (NaCl), common table salt. Sodium (Na) readily loses one electron to achieve stability, while chlorine readily gains that electron. The electrostatic attraction between the positively charged sodium ion (Na⁺) and the negatively charged chloride ion (Cl⁻) constitutes the ionic bond.
Chlorine's reactivity also extends to nonmetals. While chlorine's preference is to gain an electron, it can also share electrons with other nonmetals, forming covalent bonds. In covalent bonding, atoms share electrons to achieve a stable octet. This is observed in molecules like chlorine gas (Cl₂), where two chlorine atoms share one electron pair to complete each other's octets. This bond is a strong example of a single covalent bond.
Chlorine's Role in Various Applications: A testament to its Reactivity
The reactivity stemming from its seven valence electrons makes chlorine a versatile element with numerous applications across diverse fields. Here are some notable examples:
1. Disinfectant and Water Purification:
Chlorine's strong oxidizing power makes it an effective disinfectant, widely used in water treatment plants to kill harmful bacteria and viruses. The addition of chlorine to water forms hypochlorous acid (HOCl), a powerful oxidizing agent that inactivates pathogens. This ensures safe drinking water for millions worldwide. This is a direct consequence of chlorine's tendency to readily accept an electron and oxidize other substances.
2. Production of PVC (Polyvinyl Chloride):
PVC, a widely used plastic, is synthesized through the polymerization of vinyl chloride monomers. Chlorine plays a crucial role in the production of vinyl chloride, a critical building block for this versatile material used in pipes, flooring, and various other applications. The strong covalent bonds formed by chlorine contribute to the durability and stability of PVC.
3. Bleaching Agent:
Chlorine's powerful oxidizing ability is also utilized in bleaching agents. Chlorine-based bleach can remove stains and whiten fabrics by oxidizing colored compounds, effectively breaking down their chromophores. The reactive nature of chlorine in this capacity is again a direct outcome of its electron configuration.
4. Pharmaceuticals and Medicine:
Chlorine and its compounds have applications in the pharmaceutical industry. Some chlorine-containing compounds exhibit antimicrobial, antifungal, or antiviral properties. Chlorine-based drugs can target specific biological processes and have played crucial roles in the treatment of various infections. The precise way chlorine functions in these medicinal compounds is often dictated by its capacity to form both ionic and covalent bonds.
5. Industrial Processes:
Chlorine's reactivity finds use in diverse industrial processes. It acts as a reactant in various chemical syntheses, contributing to the production of numerous essential chemicals, including solvents, refrigerants, and pesticides. Its role in these applications hinges on its ability to participate in a wide range of chemical reactions, directly tied to its seven valence electrons.
Beyond the Basics: Exploring More Complex Concepts
While we’ve focused on the basic principles, exploring chlorine's behavior at a more advanced level reveals even more complexity and nuance:
-
Oxidation States: Chlorine can exhibit various oxidation states, ranging from -1 (most common, as in chloride ions) to +7 (in compounds like perchlorates). These variations are intricately linked to the ability of chlorine atoms to gain, lose, or share electrons in different chemical environments.
-
Bonding Variations: The nature of chemical bonds chlorine forms can be ionic, covalent, or even polar covalent, depending on the electronegativity difference between chlorine and the bonding partner. These nuances in bonding contribute to the wide range of chemical properties observed in chlorine compounds.
-
Reactivity Trends: Chlorine's reactivity within its group (halogens) follows predictable trends related to electronegativity and atomic size. Understanding these trends allows scientists to predict the reactivity of other halogen elements based on their electronic configurations.
Conclusion: A Comprehensive Understanding of Chlorine's Valence Electrons
In conclusion, chlorine's seven valence electrons are not simply a number in a textbook; they are the fundamental key to understanding its chemical behavior, its wide range of applications, and its overall importance in various scientific and industrial fields. This understanding, gained by examining its electronic configuration and bonding tendencies, allows us to appreciate the intricate role this element plays in our world. From the simple act of purifying water to the complex synthesis of pharmaceuticals, chlorine’s reactivity, a direct consequence of its seven valence electrons, continues to shape our lives in significant ways. Further research into the nuances of chlorine's reactivity will undoubtedly lead to even more innovative and crucial applications in the future.
Latest Posts
Latest Posts
-
Lowest Common Multiple Of 36 And 45
Mar 24, 2025
-
What Is Pi As A Fraction
Mar 24, 2025
-
How Many Kilograms Is 185 Lbs
Mar 24, 2025
-
What Is The Lcm Of 10 15
Mar 24, 2025
-
480 Cm Is Equal To How Many Meters
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
