Nucleotides Are Attached By Bonds Between The
listenit
Mar 17, 2025 · 7 min read
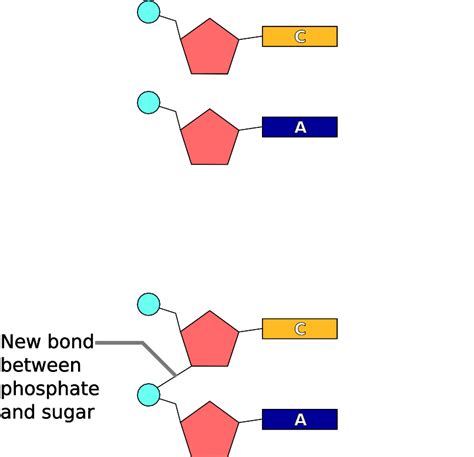
Table of Contents
Nucleotides: The Building Blocks of Life and the Bonds That Unite Them
Nucleotides are the fundamental building blocks of nucleic acids, the vital molecules that carry genetic information (DNA and RNA). Understanding how these nucleotides are linked is crucial to comprehending the structure, function, and replication of DNA and RNA. This article delves deep into the fascinating world of nucleotide bonding, exploring the types of bonds involved, their significance in the overall structure of nucleic acids, and the implications of these bonds for cellular processes.
The Nucleotide Structure: A Quick Recap
Before diving into the intricacies of nucleotide bonding, let's briefly review the structure of a nucleotide itself. Each nucleotide consists of three components:
-
A nitrogenous base: These are organic molecules containing nitrogen and are classified into two main groups: purines (adenine – A, and guanine – G) and pyrimidines (cytosine – C, thymine – T, and uracil – U). Thymine is found only in DNA, while uracil replaces thymine in RNA.
-
A pentose sugar: This is a five-carbon sugar molecule. In DNA, it's deoxyribose, while in RNA, it's ribose. The difference lies in the presence of a hydroxyl (-OH) group on the 2' carbon of ribose, which is absent in deoxyribose. This seemingly minor difference has profound consequences for the stability and functionality of the two nucleic acids.
-
A phosphate group: This is a negatively charged group consisting of a phosphorus atom bonded to four oxygen atoms. It's the phosphate group that provides the acidic nature of nucleic acids and plays a crucial role in energy transfer within the cell.
The Phosphodiester Bond: The Backbone of Nucleic Acids
The nucleotides are linked together to form the polynucleotide chains of DNA and RNA through phosphodiester bonds. This bond is formed between the 3'-hydroxyl group (-OH) of one nucleotide's sugar and the 5'-phosphate group of the next nucleotide. This linkage is crucial because it dictates the directionality of the polynucleotide chain. The chain has a 5' end (with a free phosphate group) and a 3' end (with a free hydroxyl group). This 5' to 3' orientation is fundamental to DNA replication and transcription.
The formation of a phosphodiester bond is a dehydration reaction, meaning a water molecule is released during the process. This reaction is catalyzed by enzymes called DNA polymerases (for DNA synthesis) and RNA polymerases (for RNA synthesis). The energy required for this reaction often comes from the hydrolysis of high-energy phosphate bonds in molecules like ATP.
The significance of the phosphodiester bond:
- Structural integrity: The phosphodiester backbone provides the structural framework for the nucleic acid molecule, giving it strength and stability.
- Negative charge: The negatively charged phosphate groups contribute to the overall negative charge of DNA and RNA, affecting their interactions with proteins and other molecules.
- Hydrophilic nature: The sugar-phosphate backbone is hydrophilic (water-loving), meaning it interacts favorably with water molecules, which is essential for the solubility and stability of nucleic acids in the aqueous environment of the cell.
- Directionality: The 5' to 3' directionality of the backbone is critical for the mechanism of DNA replication and transcription.
Hydrogen Bonds: The Key to Base Pairing
While the phosphodiester bond forms the structural backbone of DNA and RNA, it is the hydrogen bonds between the nitrogenous bases that determine the specificity of base pairing and, consequently, the genetic information encoded within the molecule. These hydrogen bonds are relatively weak compared to covalent bonds, which allows for the separation of DNA strands during replication and transcription.
Specific base pairing rules:
In DNA, adenine (A) forms two hydrogen bonds with thymine (T), and guanine (G) forms three hydrogen bonds with cytosine (C). This is known as complementary base pairing. The specific number of hydrogen bonds between the base pairs influences the stability of the DNA double helix. The higher number of hydrogen bonds between G and C contributes to the higher melting temperature of DNA regions rich in G-C content.
In RNA, the base pairing rules are slightly different. Adenine (A) still pairs with uracil (U) via two hydrogen bonds, while guanine (G) continues to pair with cytosine (C) via three hydrogen bonds. The presence of uracil in RNA instead of thymine is another key difference between DNA and RNA.
The importance of hydrogen bonding in DNA and RNA:
- Specificity of base pairing: The precise hydrogen bonding ensures that only the correct bases pair, maintaining the integrity of the genetic code.
- Double helix stability: In DNA, hydrogen bonding between complementary bases contributes significantly to the stability of the double helix structure.
- DNA replication and transcription: The ability of hydrogen bonds to break and reform allows for the separation of DNA strands during replication and the unwinding of the DNA double helix during transcription.
- RNA secondary structure: Hydrogen bonding also plays a critical role in the formation of secondary structures in RNA molecules, such as hairpin loops and stem-loops, which are important for RNA function.
Glycosidic Bond: Linking Base and Sugar
Another crucial bond in nucleotide structure is the glycosidic bond. This covalent bond connects the nitrogenous base to the 1'-carbon atom of the pentose sugar. The glycosidic bond's orientation is important in determining the molecule's overall conformation and interactions with other molecules. The bond is formed between the N9 of purines and the C1' of the sugar, while in pyrimidines the bond is formed between the N1 of pyrimidines and the C1' of the sugar.
The glycosidic bond's stability is crucial for maintaining the structural integrity of the nucleotide and subsequently the nucleic acid polymer. It's resistant to hydrolysis under normal physiological conditions, ensuring the long-term stability of genetic information.
Other Important Interactions
Beyond the core bonds mentioned above, several other interactions contribute to the overall structure and stability of nucleic acids:
- Base stacking: Base stacking interactions are non-covalent interactions between adjacent base pairs within the DNA double helix. These interactions contribute to the stability of the DNA double helix.
- Hydrophobic interactions: The hydrophobic nature of the nitrogenous bases favors their clustering in the interior of the DNA double helix, away from the surrounding water molecules. This hydrophobic effect significantly contributes to the stability of the double helix structure.
- Ion-dipole interactions: The negatively charged phosphate backbone interacts favorably with positively charged ions (cations), like Mg²⁺, which helps to neutralize the negative charge and stabilize the DNA structure.
Implications for Cellular Processes
The specific types of bonds connecting nucleotides have profound implications for various cellular processes:
- DNA Replication: The ability of hydrogen bonds to break and reform allows for the separation of DNA strands, enabling the accurate replication of genetic information. The phosphodiester bond provides the structural framework for the new DNA strands being synthesized.
- Transcription: Similarly, the breaking and reforming of hydrogen bonds between DNA strands allow for the transcription of genetic information into RNA molecules. The phosphodiester bond forms the backbone of the newly synthesized RNA molecule.
- Translation: The sequence of nucleotides in mRNA dictates the amino acid sequence of proteins. The stability of the mRNA molecule depends, in part, on the strength of its phosphodiester bonds and its secondary structure stabilized by hydrogen bonds.
- DNA Repair: The specificity of base pairing is critical for DNA repair mechanisms, which can correct errors in the DNA sequence. The ability to recognize mismatched base pairs relies on the hydrogen-bonding properties of the bases.
Conclusion
The bonds connecting nucleotides—phosphodiester bonds, hydrogen bonds, and glycosidic bonds—are not just passive links; they are integral players in the dynamic world of cellular processes. Their properties, strengths, and specificities dictate the structural integrity, stability, and functionality of DNA and RNA. Understanding these bonds is essential for appreciating the remarkable elegance and complexity of life's fundamental building blocks and the processes they support. Further research into nucleotide interactions promises to reveal even more intricate details about these crucial molecules and their roles in maintaining life's delicate balance. This knowledge is instrumental in advancements in medicine, biotechnology, and our understanding of genetics as a whole.
Latest Posts
Latest Posts
-
Chromosomes Are Duplicated During What Stage Of The Cell Cycle
Mar 18, 2025
-
During Which Phases Of Cellular Respiration Is Co2 Produced
Mar 18, 2025
-
The Level With The Most Energy Is The Level
Mar 18, 2025
-
How Many Oz Is 500 Ml Of Water
Mar 18, 2025
-
7 5a 4 1 14 8a
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Nucleotides Are Attached By Bonds Between The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
