Negatively Charged Particle In The Atom
listenit
Mar 15, 2025 · 7 min read
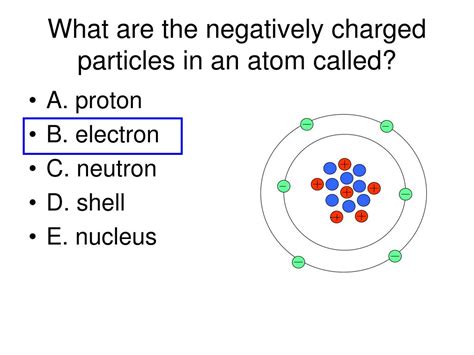
Table of Contents
Negatively Charged Particles in the Atom: A Deep Dive into Electrons
The atom, the fundamental building block of matter, is a fascinating microcosm of activity. While often simplified as a miniature solar system, its complexity and intricacies continue to captivate scientists and researchers. At the heart of this complexity lies the negatively charged particle: the electron. This article delves deep into the world of electrons, exploring their properties, behavior, and significance in various scientific fields.
Understanding the Electron: A Subatomic Particle
The electron, denoted by the symbol 'e⁻', is a fundamental subatomic particle. This means it's not composed of smaller constituents; it's considered one of the elementary particles of the Standard Model of particle physics. It carries a single negative elementary electric charge, approximately −1.602 × 10⁻¹⁹ coulombs. This negative charge is crucial in determining the electron's interactions with other particles and its role in chemical bonding and electrical phenomena.
Key Properties of Electrons:
-
Mass: Electrons have a remarkably small mass, approximately 9.109 × 10⁻³¹ kilograms. This is far smaller than the mass of protons and neutrons, the other major constituents of atoms. Their minuscule mass significantly influences their behavior and movement within the atom.
-
Charge: As mentioned earlier, electrons possess a negative elementary charge. This charge is equal in magnitude but opposite in sign to the positive charge of a proton. This balance of charges within an atom is fundamental to its overall neutrality.
-
Spin: Electrons exhibit an intrinsic angular momentum, known as spin. This is a quantum mechanical property, and it's not a literal spinning motion. Instead, it's an inherent characteristic that contributes to the electron's magnetic moment and influences its behavior in magnetic fields. The spin of an electron is quantized, meaning it can only have specific values, typically represented as +½ or −½.
-
Wave-Particle Duality: Electrons display a remarkable wave-particle duality. This means they exhibit properties of both waves and particles. They can behave like particles, possessing mass and charge, but they also exhibit wave-like behavior, such as diffraction and interference, as demonstrated by the famous double-slit experiment.
-
Quantum Numbers: Electrons are described by a set of four quantum numbers that define their state within an atom: principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms). These numbers determine the electron's energy level, orbital shape, spatial orientation, and spin.
The Electron's Role in Atomic Structure
The electron's presence and behavior are critical in defining the structure and properties of an atom. Electrons occupy specific energy levels or orbitals surrounding the atom's nucleus, which contains positively charged protons and neutral neutrons. The arrangement of electrons in these orbitals determines the atom's chemical properties and how it interacts with other atoms.
Electron Shells and Subshells:
Electrons are arranged in shells, representing distinct energy levels. Each shell can accommodate a specific number of electrons. The innermost shell (n=1) can hold a maximum of two electrons, while subsequent shells hold progressively more electrons. Within each shell are subshells, further subdividing the energy levels and defining the shape of the electron orbitals (s, p, d, f). The filling of these shells and subshells follows specific rules, governed by the Pauli Exclusion Principle and Hund's rule.
Electron Configuration and Chemical Properties:
The electron configuration of an atom describes the distribution of electrons in its various energy levels and orbitals. This configuration dictates the atom's chemical behavior. Atoms tend to react chemically to achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their outermost shell (valence shell). This drive for stability underlies the formation of chemical bonds and the existence of molecules. The number of valence electrons, particularly, determines the atom's bonding capacity and reactivity.
Electron Behavior and Interactions
Electrons don't simply sit statically in their orbitals; they are constantly in motion and interact with various forces. Understanding this dynamic behavior is crucial in comprehending atomic phenomena.
Electron Movement and Orbitals:
Electrons don't follow defined paths like planets around the sun. Instead, their location is described probabilistically using orbitals. An orbital represents the region of space where there's a high probability of finding an electron. These orbitals have distinct shapes and energy levels, reflecting the quantum nature of the electron.
Electron Interactions with Electromagnetic Fields:
Electrons, possessing both charge and spin, interact strongly with electromagnetic fields. External magnetic fields can affect the electron's spin and orbital motion, leading to phenomena like Zeeman splitting, where spectral lines are split into multiple components in the presence of a magnetic field. Similarly, electric fields can alter the electron's trajectory and energy levels.
Electron Emission and Absorption:
Electrons can absorb energy and jump to higher energy levels (excited states). Conversely, when an electron transitions from a higher energy level to a lower one, it emits energy, often in the form of light (photons). This process forms the basis of spectroscopy, a technique used to analyze the composition of matter by examining the emitted or absorbed light.
Electrons in Different Scientific Fields
The importance of electrons extends far beyond the realm of atomic structure. Their unique properties and behaviors play vital roles in various scientific and technological fields.
Chemistry:
Electrons are the cornerstone of chemical bonding. The interactions between electrons in different atoms lead to the formation of covalent, ionic, and metallic bonds, creating the diverse range of molecules and materials in the world around us. Understanding electron behavior is crucial for predicting chemical reactions and designing new materials with specific properties.
Physics:
Electrons are crucial in many areas of physics, including condensed matter physics (studying the behavior of electrons in solids), nuclear physics (understanding electron capture and beta decay), and particle physics (exploring the fundamental nature of electrons and their interactions with other particles).
Electronics and Technology:
Electrons are the foundation of modern electronics. The flow of electrons through conductors forms the basis of electric current, powering countless devices, from simple light bulbs to complex computers. The manipulation of electron flow through semiconductors is crucial in transistors, integrated circuits, and other electronic components.
Materials Science:
The properties of materials are strongly influenced by their electron configurations and interactions. Materials scientists leverage an understanding of electron behavior to design new materials with tailored properties for various applications, such as high-strength alloys, superconductors, and advanced semiconductors.
The Ongoing Exploration of Electrons
Despite extensive research and understanding, the electron continues to be a source of fascination and ongoing investigation. The precise nature of its internal structure and behavior remains an active area of research in particle physics. Further advancements in our understanding of electrons are expected to lead to breakthroughs in various fields, fostering new technologies and providing deeper insights into the fundamental workings of the universe. Research continues to refine our understanding of electron interactions, leading to improved theoretical models and predictions, which in turn, drive innovation across various scientific and technological sectors.
Future Directions in Electron Research:
-
Advanced Materials: Research into manipulating electron behavior in novel materials is paving the way for new technologies like high-temperature superconductors, efficient solar cells, and advanced electronic devices.
-
Quantum Computing: Electrons’ quantum properties are being harnessed for the development of quantum computers, promising unprecedented computational power.
-
Fundamental Physics: Ongoing research into the fundamental properties of electrons, such as their possible internal structure and potential interactions with other yet-undiscovered particles, will continue to challenge and expand our understanding of the universe.
In conclusion, the negatively charged particle within the atom, the electron, is far more than a simple subatomic particle. It is a fundamental component of matter, playing a critical role in shaping atomic structure, chemical bonding, and the technological advancements that underpin modern society. The ongoing exploration of electrons promises to unveil further insights into the intricacies of the universe and drive innovations in diverse fields for years to come. Its study remains a cornerstone of scientific progress and a testament to the enduring power of human curiosity and scientific inquiry.
Latest Posts
Latest Posts
-
What Is Square Root Of 34
Mar 15, 2025
-
What Is An Equivalent Fraction For 3 8
Mar 15, 2025
-
Is A Rhombus Sometimes A Rectangle
Mar 15, 2025
-
How Many Feet Are In 240 Inches
Mar 15, 2025
-
What Is The Equivalent Fraction For 5 8
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Negatively Charged Particle In The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
