Mass Of An Electron In Grams
listenit
Mar 31, 2025 · 6 min read
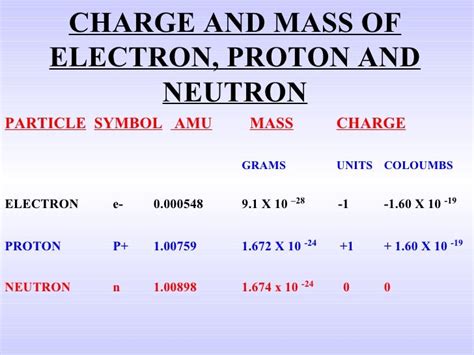
Table of Contents
The Mass of an Electron in Grams: A Deep Dive into Subatomic Physics
The electron, a fundamental particle of matter, holds a crucial place in our understanding of the universe. While seemingly insignificant individually, the collective behavior of electrons dictates the properties of atoms, molecules, and ultimately, all matter. One of the key characteristics of an electron is its mass, a quantity often expressed in kilograms or atomic mass units (amu). However, understanding the electron's mass in grams offers a unique perspective, bridging the gap between the abstract world of quantum mechanics and the tangible world of everyday experience. This article will delve into the intricacies of determining and understanding the electron's mass in grams, exploring related concepts and highlighting its significance.
Understanding the Electron's Mass
The mass of an electron is incredibly small, far beyond the scope of everyday measurement. This tiny mass is responsible for several crucial phenomena, including electric current, chemical bonding, and the behavior of materials in electromagnetic fields. Its precise measurement has been a significant undertaking in the field of physics, requiring sophisticated experimental techniques.
Defining Mass in Physics
Before diving into the electron's mass, it's essential to clarify the concept of mass in physics. Mass is a fundamental property of matter that reflects its resistance to acceleration. In simpler terms, it's a measure of how difficult it is to change an object's velocity. This property is distinct from weight, which is the force exerted on an object due to gravity. While weight can change depending on the gravitational field, mass remains constant.
The Electron's Mass in Kilograms
The most commonly used value for the electron's rest mass (the mass when it's not moving at relativistic speeds) is approximately 9.10938356 × 10⁻³¹ kilograms. This incredibly small number emphasizes just how minuscule the electron is.
Converting to Grams: A Practical Perspective
To convert the electron's mass from kilograms to grams, we simply multiply by 1000 (since there are 1000 grams in a kilogram):
9.10938356 × 10⁻³¹ kg * 1000 g/kg = 9.10938356 × 10⁻²⁸ grams
This figure, while still astronomically small, provides a more relatable perspective. Imagine trying to weigh this minuscule amount – it's far beyond the capabilities of even the most sensitive laboratory scales.
Methods for Determining the Electron's Mass
Determining the electron's mass has been a monumental task in physics, requiring ingenious experimental techniques and advancements in technology. Some of the crucial methods include:
Millikan's Oil Drop Experiment
While not directly measuring the electron's mass, Robert Millikan's famous oil drop experiment (conducted in the early 20th century) was instrumental in determining the charge of an electron. Knowing the charge and using other experimental data involving the electron's motion in electric and magnetic fields allowed scientists to calculate its mass. This experiment laid the groundwork for more accurate measurements.
Thomson's Cathode Ray Tube Experiment
J.J. Thomson's experiments with cathode ray tubes played a crucial role in the discovery of the electron itself. By observing the deflection of cathode rays (streams of electrons) in electric and magnetic fields, Thomson was able to determine the charge-to-mass ratio (e/m) of the electron. This was a significant step towards determining the electron's mass, albeit indirectly.
Spectroscopic Techniques
Modern spectroscopic techniques, employing highly sensitive instruments, provide incredibly precise measurements of the electron's mass. These techniques analyze the energy levels of atoms and molecules, which are directly influenced by the electron's mass and its interactions with other particles. The precision of these measurements has steadily improved over the years, leading to the highly accurate value we use today.
The Significance of the Electron's Mass
The seemingly minuscule mass of the electron has far-reaching consequences across various fields of science and technology:
Chemistry and Molecular Bonding
The electron's mass influences the strength of chemical bonds. The electrons involved in bonding determine the properties of molecules, influencing their reactivity, stability, and overall behavior. Without the electron's mass, the intricate world of chemical reactions and molecular structures would simply not exist.
Electrical Conductivity
The flow of electrons is what constitutes electric current. The electron's mass affects the ease with which electrons can move through a material, influencing the material's conductivity. Materials with low electron mobility are poor conductors, while those with high electron mobility are good conductors.
Magnetism
Electrons possess an intrinsic property called spin, which generates a magnetic field. The electron's mass, combined with its spin, plays a fundamental role in magnetism. The collective magnetic moments of electrons in a material contribute to its overall magnetic properties.
Relativistic Effects and the Electron's Mass
At very high speeds, approaching the speed of light, the electron's mass increases due to relativistic effects, as described by Einstein's theory of special relativity. This increase in mass is not an increase in the intrinsic property of the electron but rather a consequence of its increased kinetic energy. This effect becomes significant only at speeds close to the speed of light and is crucial in high-energy physics experiments.
The Electron's Mass and the Standard Model
The electron's mass is a fundamental parameter in the Standard Model of particle physics, a theoretical framework that describes the fundamental constituents of matter and their interactions. While the Standard Model successfully explains many phenomena, it doesn't explain the precise value of the electron's mass. This is one of the open questions in particle physics, prompting ongoing research and theoretical investigations.
Conclusion: A Tiny Particle, a Vast Impact
The electron's mass, though seemingly insignificant in everyday life when expressed in grams (9.10938356 × 10⁻²⁸ grams), plays a pivotal role in shaping the universe as we know it. Its precise measurement, a testament to human ingenuity and scientific progress, continues to refine our understanding of fundamental physics. From the intricate dance of electrons in chemical bonds to the powerful forces in high-energy physics experiments, the electron’s tiny mass has an immense impact on the world around us. Further research into the nature of the electron and its mass promises to unlock even deeper insights into the fundamental laws governing our universe. The quest to understand this minuscule particle underscores the remarkable power of scientific inquiry and the ongoing quest to unravel the mysteries of the cosmos. The exploration of the electron's mass, therefore, remains a vibrant and crucial area of research, pushing the boundaries of our scientific knowledge and technological capabilities. Its continued study will undoubtedly reveal further profound insights into the workings of the universe.
Latest Posts
Latest Posts
-
What Is 5 Divided By 1 4
Apr 01, 2025
-
150 Rounded To The Nearest Hundred
Apr 01, 2025
-
What Is 1 2 Of 1 2 3
Apr 01, 2025
-
A Rose Garden Is Formed By Joining A Rectangle
Apr 01, 2025
-
What Is The Fraction For 1875
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Mass Of An Electron In Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
