Lewis Diagram For A Ion With A Total Of Electrons
listenit
Mar 15, 2025 · 6 min read
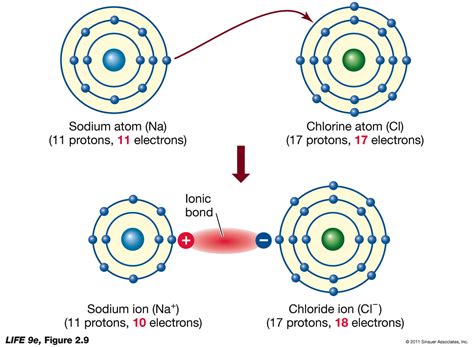
Table of Contents
Lewis Diagrams for Ions: A Comprehensive Guide
Lewis diagrams, also known as Lewis structures or electron dot diagrams, are visual representations of the valence electrons in atoms and molecules. They are invaluable tools for understanding chemical bonding, predicting molecular geometry, and comprehending the reactivity of substances. While often used for neutral molecules, their application extends to ions, charged species with an unequal number of protons and electrons. This comprehensive guide will delve into the process of drawing Lewis diagrams for ions, encompassing various complexities and providing numerous examples.
Understanding Valence Electrons and Ionic Bonding
Before diving into the intricacies of drawing Lewis diagrams for ions, a firm understanding of valence electrons and ionic bonding is crucial.
Valence Electrons: The Key Players
Valence electrons are the outermost electrons of an atom. These electrons participate in chemical bonding and determine the chemical properties of an element. The number of valence electrons is directly related to an element's group number on the periodic table (excluding transition metals). For example, Group 1 elements (alkali metals) have one valence electron, Group 2 elements (alkaline earth metals) have two, and Group 17 elements (halogens) have seven.
Ionic Bonding: A Transfer of Electrons
Ionic bonding occurs when atoms transfer electrons to achieve a stable electron configuration, typically resembling a noble gas (Group 18 elements). This transfer creates ions: positively charged cations (formed by electron loss) and negatively charged anions (formed by electron gain). The electrostatic attraction between these oppositely charged ions forms the ionic bond.
Drawing Lewis Diagrams for Cations
Creating Lewis diagrams for cations involves removing electrons from the neutral atom's Lewis structure. Remember that electrons are removed from the highest energy level (valence shell) first.
Example 1: Sodium Ion (Na⁺)
Sodium (Na) has 11 electrons and 1 valence electron. To form a +1 ion (Na⁺), it loses one electron.
- Draw the Lewis structure for neutral sodium: Na •
- Remove one electron: Na⁺ (no dots)
Example 2: Magnesium Ion (Mg²⁺)
Magnesium (Mg) has 12 electrons and 2 valence electrons. To form a +2 ion (Mg²⁺), it loses two electrons.
- Draw the Lewis structure for neutral magnesium: Mg ••
- Remove two electrons: Mg²⁺ (no dots)
Example 3: Aluminum Ion (Al³⁺)
Aluminum (Al) has 13 electrons and 3 valence electrons. To form a +3 ion (Al³⁺), it loses three electrons.
- Draw the Lewis structure for neutral aluminum: Al •••
- Remove three electrons: Al³⁺ (no dots)
Drawing Lewis Diagrams for Anions
Creating Lewis diagrams for anions involves adding electrons to the neutral atom's Lewis structure. These added electrons occupy available spaces in the valence shell, often completing the octet (eight electrons).
Example 1: Chloride Ion (Cl⁻)
Chlorine (Cl) has 17 electrons and 7 valence electrons. To form a -1 ion (Cl⁻), it gains one electron.
- Draw the Lewis structure for neutral chlorine: Cl •••••••
- Add one electron: Cl [•••••••]⁻ (Note the square brackets and the negative charge indicating the ion)
Example 2: Oxide Ion (O²⁻)
Oxygen (O) has 8 electrons and 6 valence electrons. To form a -2 ion (O²⁻), it gains two electrons.
- Draw the Lewis structure for neutral oxygen: O •••••
- Add two electrons: O [••••••]²⁻
Example 3: Sulfide Ion (S²⁻)
Sulfur (S) has 16 electrons and 6 valence electrons. To form a -2 ion (S²⁻), it gains two electrons.
- Draw the Lewis structure for neutral sulfur: S •••••
- Add two electrons: S [••••••]²⁻
Lewis Diagrams for Polyatomic Ions
Polyatomic ions are charged groups of atoms covalently bonded together. Drawing Lewis diagrams for these ions requires a slightly more involved approach.
Example 1: Hydroxide Ion (OH⁻)
- Count valence electrons: Oxygen (6) + Hydrogen (1) + 1 (from the -1 charge) = 8 valence electrons
- Arrange atoms: The hydrogen atom is typically bonded to the oxygen atom.
- Distribute electrons: Place electron pairs to satisfy the octet rule for oxygen. Hydrogen only requires two electrons.
[ O-H ]⁻ - Check the formal charges: Ensure the formal charges of each atom add up to the overall charge of the ion.
Example 2: Nitrate Ion (NO₃⁻)
- Count valence electrons: Nitrogen (5) + Oxygen (6 x 3) + 1 (from the -1 charge) = 24 valence electrons
- Arrange atoms: Nitrogen is typically the central atom, surrounded by three oxygen atoms.
- Distribute electrons: Form single bonds between nitrogen and each oxygen. Complete the octets of oxygen atoms. This will leave one electron pair to be placed on the central nitrogen atom. However, since we have 24 electrons and only 22 have been placed by now, we add one double bond between oxygen and Nitrogen in order to achieve 24 electrons in total and satisfy octet rule.
There are different resonance structures for Nitrate ion and all the oxygen atoms are chemically equivalent in Nitrate ion.[ O=N-O⁻ ]⁻
Example 3: Ammonium Ion (NH₄⁺)
- Count valence electrons: Nitrogen (5) + Hydrogen (1 x 4) - 1 (from the +1 charge) = 8 valence electrons.
- Arrange atoms: Nitrogen as the central atom, surrounded by four hydrogen atoms.
- Distribute electrons: Create single bonds between nitrogen and each hydrogen atom.
All the hydrogen atoms are chemically equivalent.[H-N-H⁺ | H]⁺
Formal Charge Calculation
To ensure your Lewis structure is accurate, calculate the formal charge of each atom. The formal charge is the difference between the number of valence electrons in a free atom and the number of electrons assigned to that atom in the Lewis structure. The formula is:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 Bonding Electrons)
A well-drawn Lewis structure will have formal charges as close to zero as possible, and the sum of formal charges will equal the overall charge of the ion.
Exceptions to the Octet Rule
Some molecules and ions don't follow the octet rule. These exceptions include:
- Electron-deficient molecules: These molecules have fewer than eight electrons around the central atom (e.g., BeCl₂).
- Odd-electron molecules (radicals): These molecules have an odd number of valence electrons, resulting in an unpaired electron (e.g., NO₂).
- Expanded octet molecules: These molecules have more than eight electrons around the central atom (common with elements in period 3 or higher, e.g., SF₆).
Drawing Lewis structures for these exceptions requires careful consideration of the specific atoms involved and their ability to accommodate more or fewer electrons than the octet rule dictates.
Importance of Lewis Diagrams in Chemistry
Lewis diagrams are fundamental to understanding various aspects of chemistry:
- Predicting molecular geometry: The arrangement of electron pairs (bonding and non-bonding) influences a molecule's shape.
- Understanding chemical reactivity: Lewis diagrams help predict how molecules will react based on their electron distribution.
- Explaining bonding types: They clearly depict covalent and coordinate covalent bonds.
- Illustrating resonance structures: For molecules with multiple possible Lewis structures, resonance structures can show the delocalization of electrons.
Conclusion
Mastering the art of drawing Lewis diagrams for ions is essential for any student or professional working in chemistry. This guide provided a detailed and comprehensive walkthrough, from the fundamentals of valence electrons and ionic bonding to the complexities of polyatomic ions and exceptions to the octet rule. By understanding these concepts and practicing extensively, you can develop a strong foundation for tackling more advanced chemical concepts. Remember to always count your electrons carefully and utilize formal charge calculations to verify the accuracy of your Lewis diagrams. Through consistent practice and application, the seemingly complex process of drawing Lewis diagrams will become second nature, opening up a world of chemical understanding.
Latest Posts
Latest Posts
-
C 5 9 F 32 For F
Mar 15, 2025
-
What Is The Top Of A Wave Called
Mar 15, 2025
-
Log X 3 Log X 1
Mar 15, 2025
-
8x 4 4x 3 4 6x 4 4
Mar 15, 2025
-
What Do The Arrows On A Food Chain Represent
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Lewis Diagram For A Ion With A Total Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
