Isotopes Are Atoms Of The Same Element That Have Different
listenit
Mar 22, 2025 · 7 min read
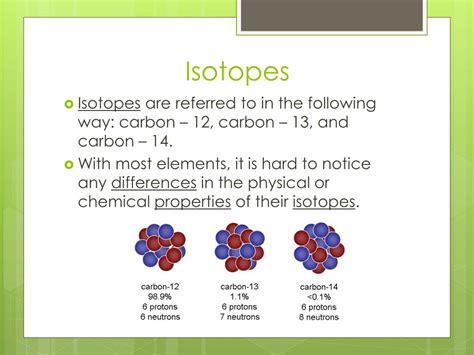
Table of Contents
Isotopes: Atoms of the Same Element, Different Numbers of Neutrons
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This seemingly subtle difference has profound implications across various scientific fields, impacting everything from nuclear medicine to geological dating and our understanding of the universe's origins. Let's delve deeper into the fascinating world of isotopes, exploring their properties, applications, and significance.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we explore isotopes, it's crucial to understand the basic building blocks of an atom. An atom comprises three fundamental subatomic particles:
-
Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; for instance, all atoms with one proton are hydrogen, while those with six protons are carbon. This number is known as the atomic number.
-
Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Unlike protons, the number of neutrons in an atom can vary without changing the element's identity.
-
Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom, balancing the positive charge of the protons.
What Makes Isotopes Different?
Isotopes of the same element share the same number of protons but differ in their number of neutrons. This difference in neutron number alters the atom's mass but not its chemical properties significantly. The mass number of an atom is the sum of its protons and neutrons. Different isotopes of the same element will have the same atomic number but different mass numbers.
For example, consider carbon:
- Carbon-12 (¹²C): 6 protons and 6 neutrons (mass number = 12)
- Carbon-13 (¹³C): 6 protons and 7 neutrons (mass number = 13)
- Carbon-14 (¹⁴C): 6 protons and 8 neutrons (mass number = 14)
All three are isotopes of carbon because they all have 6 protons. However, they vary in their neutron count and, consequently, their mass. This seemingly small difference has significant consequences.
Isotope Notation and Representation
Isotopes are typically represented using a specific notation that clearly indicates the element and its mass number. The notation follows this format:
^A_Z X
Where:
- X is the element's chemical symbol (e.g., C for carbon, U for uranium).
- Z is the atomic number (number of protons).
- A is the mass number (number of protons + neutrons).
For example, Carbon-14 would be written as ¹⁴₆C.
Properties of Isotopes: Similarities and Differences
While isotopes of an element share the same number of protons and thus the same chemical behavior in most reactions, differences arise due to variations in neutron number:
-
Mass: The most obvious difference is their mass. Heavier isotopes have more neutrons and therefore a greater mass.
-
Nuclear Stability: The ratio of protons to neutrons greatly influences an isotope's stability. Some isotopes are stable, meaning they don't spontaneously decay. Others are unstable or radioactive, undergoing radioactive decay to achieve a more stable configuration. This decay process emits radiation in various forms (alpha, beta, gamma).
-
Nuclear Spin: The number of neutrons affects the nuclear spin of an atom, influencing its interaction with magnetic fields. This property is exploited in techniques like nuclear magnetic resonance (NMR) spectroscopy.
Applications of Isotopes: A Wide-Ranging Impact
The unique properties of isotopes have led to their extensive use across various fields:
1. Nuclear Medicine: Diagnosis and Treatment
Radioactive isotopes, often called radioisotopes, play a vital role in medical imaging and treatment. Techniques like PET (positron emission tomography) scans utilize radioisotopes that emit positrons, allowing doctors to visualize metabolic activity in the body and detect diseases like cancer at an early stage. Radioisotopes are also used in radiotherapy, targeting cancerous cells and destroying them with radiation. Examples include iodine-131 for thyroid cancer treatment and cobalt-60 for external beam radiation therapy.
2. Geological Dating: Unveiling Earth's History
Radioactive isotopes are essential tools in radiometric dating, enabling scientists to determine the age of rocks, fossils, and other geological materials. By measuring the ratio of parent isotopes to their decay products, researchers can estimate the time elapsed since the sample's formation. The most well-known example is carbon-14 dating, used to date organic materials up to around 50,000 years old. Other isotopes like uranium-238 and potassium-40 are employed to date older geological formations.
3. Industrial Applications: Tracing and Monitoring
Isotopes serve as valuable tracers in various industrial processes. They allow scientists to track the movement of materials, monitor flow rates, and detect leaks in pipelines or other systems. This technology finds applications in diverse industries, including manufacturing, environmental monitoring, and oil exploration.
4. Agricultural Research: Studying Nutrient Uptake
Isotopes are used to study nutrient uptake and metabolism in plants. By introducing labeled isotopes of nutrients (e.g., nitrogen-15), researchers can track their movement within the plant, optimizing fertilizer application and improving crop yields.
5. Environmental Science: Studying Pollution and Migration
Isotopes help scientists understand pollution sources and migration pathways in the environment. By analyzing isotope ratios in water, soil, or air samples, they can trace pollutants back to their origins and monitor their spread. This is crucial in managing pollution and protecting ecosystems.
Isotope Separation: Techniques and Challenges
Separating isotopes of the same element is a challenging process because they possess almost identical chemical properties. Several techniques are employed, each with its own advantages and disadvantages:
-
Gaseous Diffusion: This method relies on the slightly different diffusion rates of gaseous isotopes through a porous membrane. Heavier isotopes diffuse more slowly than lighter ones.
-
Gas Centrifugation: This technique uses high-speed centrifuges to separate isotopes based on their mass difference. Heavier isotopes migrate towards the outer edge of the centrifuge.
-
Laser Isotope Separation: This sophisticated method uses lasers tuned to specific wavelengths to selectively excite and ionize a particular isotope, allowing its separation from others.
-
Electromagnetic Separation: This technique utilizes strong magnetic and electric fields to separate isotopes based on their mass-to-charge ratio.
Isotopes and Nuclear Reactions: Fission and Fusion
Isotopes play a pivotal role in nuclear reactions, particularly fission and fusion:
-
Nuclear Fission: The process of splitting a heavy atomic nucleus (like uranium-235) into lighter nuclei, releasing a tremendous amount of energy. This process is harnessed in nuclear power plants and nuclear weapons.
-
Nuclear Fusion: The process of combining light atomic nuclei (like deuterium and tritium, isotopes of hydrogen) to form a heavier nucleus, releasing even greater energy than fission. Fusion is the energy source of stars and is currently being researched as a potential clean energy source on Earth.
The Significance of Isotopes in Scientific Research
The study of isotopes has been instrumental in advancing our understanding of various scientific disciplines:
-
Cosmology: Isotope ratios in meteorites provide insights into the early solar system's formation and the processes that led to the planets' creation.
-
Climate Science: Isotope ratios in ice cores and tree rings serve as proxies for past climate conditions, helping scientists reconstruct long-term climate patterns and understand climate change.
-
Archaeology: Isotope analysis helps archaeologists understand ancient diets, migration patterns, and the environmental context of past civilizations.
-
Medicine: Isotope tracers help researchers study complex metabolic pathways and drug interactions, leading to the development of new diagnostic tools and treatments.
Conclusion: A World Shaped by Isotopes
Isotopes, despite their seemingly minor differences in neutron numbers, have a profound impact on our world. From enabling medical breakthroughs to revealing Earth's ancient history and powering our technology, they are fundamental to numerous scientific advancements. Continued research into isotopes promises further breakthroughs and a deeper understanding of our universe and the processes shaping it. The versatility and significance of isotopes underscore their crucial role in scientific progress and technological innovation, making them a continuing area of fascinating study and application.
Latest Posts
Latest Posts
-
What Is 0 6 As A Fraction
Mar 23, 2025
-
How Many Mcg In A Kg
Mar 23, 2025
-
What Is The Gcf Of 36 And 45
Mar 23, 2025
-
40 Out Of 50 Is What Percent
Mar 23, 2025
-
What Percent Of 20 Is 6
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Are Atoms Of The Same Element That Have Different . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
