Is Water Boiling A Physical Change
listenit
Mar 13, 2025 · 6 min read
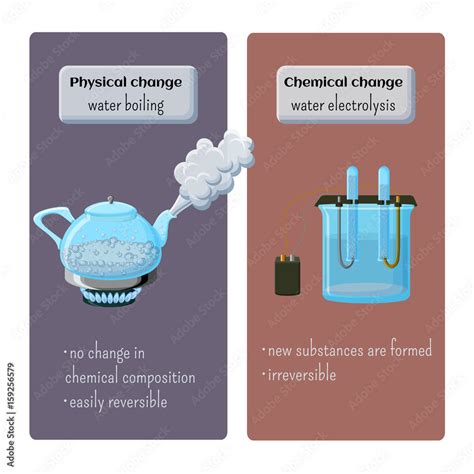
Table of Contents
Is Boiling Water a Physical Change? A Deep Dive into Phase Transitions
Boiling water is a common everyday occurrence, yet the underlying scientific principles can be surprisingly nuanced. The question, "Is boiling water a physical change?" appears simple, but a complete understanding requires exploring the nature of phase transitions, the properties of water, and the role of energy in these transformations. The short answer is yes, boiling water is a physical change, but let's delve deeper to appreciate the complexities and subtleties involved.
Understanding Physical and Chemical Changes
Before we analyze the boiling process, let's define the key terms:
-
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties, like shape, size, or state, are modified. Examples include melting ice, dissolving sugar in water, or cutting a piece of wood. These changes are often reversible.
-
Chemical Change: A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into entirely new substances with different chemical properties. This process often involves breaking and forming chemical bonds. Examples include burning wood, rusting iron, or cooking an egg. These changes are typically irreversible.
The Science Behind Boiling Water
Boiling water is a phase transition, specifically a transition from the liquid phase to the gaseous phase (vapor). This transformation is driven by heat energy. When heat is applied to liquid water, its molecules absorb energy, increasing their kinetic energy – the energy of motion.
Molecular Movement and Phase Transitions
Water molecules are held together by relatively strong intermolecular forces, primarily hydrogen bonds. In the liquid state, these bonds allow for a degree of movement, but the molecules are relatively close together. As heat is added, the kinetic energy of the molecules increases, overcoming the intermolecular forces. This leads to:
- Increased Molecular Motion: Molecules move more rapidly and randomly.
- Weakening of Hydrogen Bonds: The increased kinetic energy disrupts the hydrogen bonds holding the molecules together.
- Phase Transition to Gas: Eventually, the molecules gain enough energy to overcome the intermolecular forces completely, breaking free from the liquid and entering the gaseous phase as water vapor or steam.
This entire process – the transition from liquid water to water vapor – is a physical change. The chemical composition of the water remains H₂O throughout the process. No new substances are formed. The only change is the state of the water, from liquid to gas.
Boiling Point and Pressure
The boiling point of water is the temperature at which the vapor pressure of the water equals the atmospheric pressure. At standard atmospheric pressure (1 atm), water boils at 100°C (212°F). However, this boiling point can change with alterations in atmospheric pressure.
-
High Altitude Boiling: At higher altitudes, the atmospheric pressure is lower. Consequently, water boils at a lower temperature. This is why cooking times are often longer at higher elevations.
-
Pressure Cookers: Pressure cookers operate by increasing the pressure inside the pot. This raises the boiling point of water, allowing food to cook faster and more thoroughly at higher temperatures.
Evidence Supporting Boiling Water as a Physical Change
Several observations solidify the classification of boiling water as a physical change:
-
Reversibility: The process is reversible. If you condense the steam (water vapor) back into liquid water, you obtain the original substance, water (H₂O). This reversibility is a hallmark of a physical change.
-
No New Substance Formed: Throughout the boiling process, the chemical composition remains unchanged. The water molecules are still H₂O molecules, even in the gaseous state. There's no creation of new chemical compounds.
-
Separation Techniques: The process of boiling and condensation can be used as a separation technique. For example, you can separate salt from saltwater by boiling the water and collecting the condensed steam; the salt will remain behind. This again demonstrates the unchanged chemical nature of the water.
Distinguishing Boiling from Chemical Decomposition
It's crucial to differentiate boiling from chemical decomposition, which is a chemical change. While boiling involves a phase transition, decomposition involves the breakdown of a substance into two or more different substances. For instance, the electrolysis of water uses an electric current to decompose water into hydrogen and oxygen gas. This is a chemical change because new substances (H₂ and O₂) are formed. Boiling, on the other hand, only involves a change of state, not a change in chemical composition.
The Role of Energy in Phase Transitions
The boiling of water is an endothermic process, meaning it absorbs energy from its surroundings. The energy input is used to overcome the intermolecular forces holding the water molecules together in the liquid phase. This energy is not involved in breaking or forming chemical bonds within the water molecule itself.
Latent Heat of Vaporization
The amount of energy required to change one gram of liquid water to one gram of water vapor at its boiling point is called the latent heat of vaporization. This energy is not associated with a temperature change; it's solely used to overcome the intermolecular forces during the phase transition. Once the phase change is complete, the addition of further heat will increase the temperature of the steam.
Practical Applications and Everyday Examples
The understanding that boiling water is a physical change has numerous practical applications:
-
Water Purification: Boiling water can effectively kill many harmful microorganisms, rendering it safe for consumption. This process doesn't alter the chemical composition of the water; it simply eliminates biological contaminants.
-
Distillation: Distillation is a separation technique that uses boiling and condensation to purify liquids. This is based on the principle that boiling is a physical change, leaving the chemical composition of the liquid unaltered.
-
Cooking: Boiling is a common cooking method used to soften food or extract flavors. The process of boiling changes the texture and possibly the appearance of the food, but doesn't fundamentally alter its chemical composition (though chemical reactions might occur due to heat in the food itself).
-
Steam Generation: Boiling water is crucial for generating steam, which is used in various industrial processes, including power generation and sterilization.
Conclusion: Boiling Water is a Physical Change
In conclusion, boiling water is unequivocally a physical change. The process involves a phase transition from liquid to gas, driven by the absorption of energy to overcome intermolecular forces. The chemical composition of the water (H₂O) remains unchanged throughout the process, and the change is reversible through condensation. Understanding this fundamental distinction between physical and chemical changes is essential in many scientific and practical contexts. From everyday cooking to large-scale industrial processes, the principle of boiling water as a physical change underpins a wide range of applications. The seemingly simple act of boiling water provides a fascinating window into the fundamental nature of matter and energy.
Latest Posts
Latest Posts
-
16 Is What Percent Of 40
Mar 13, 2025
-
4 Of 50 Is What Percent
Mar 13, 2025
-
How Many Valence Electrons Does As Have
Mar 13, 2025
-
A Tennis Ball Is Thrown Vertically Upward
Mar 13, 2025
-
In Which Layer Of The Atmosphere Does Weather Take Place
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Is Water Boiling A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
