Is Oxygen A Solid Liquid Or A Gas
listenit
Apr 08, 2025 · 6 min read
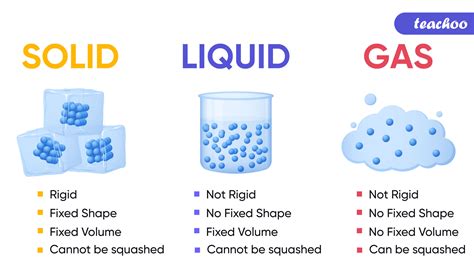
Table of Contents
Is Oxygen a Solid, Liquid, or Gas? Understanding Oxygen's States of Matter
Oxygen, a vital element for almost all life on Earth, exists in various states depending on temperature and pressure. While we commonly encounter it as a gas, understanding its ability to transition into solid and liquid forms is crucial to appreciating its multifaceted nature. This comprehensive article delves into the physical properties of oxygen across its different states, explaining the conditions under which these transitions occur and exploring the implications of these changes in various scientific fields.
Oxygen: The Gas We Breathe
In our everyday experience, oxygen exists as a gas. This is its most common state under normal atmospheric conditions. At standard temperature and pressure (STP), approximately 25°C (77°F) and 1 atmosphere, oxygen is a colorless, odorless, and tasteless gas. Its gaseous state allows it to readily mix with other gases in the atmosphere, facilitating its crucial role in respiration and combustion.
The Molecular Structure of Gaseous Oxygen
Oxygen gas exists as a diatomic molecule (O₂). This means two oxygen atoms are bonded together covalently, sharing electrons to achieve a stable electron configuration. This strong covalent bond contributes to oxygen's relatively high boiling point compared to some other diatomic gases. The weak intermolecular forces between these O₂ molecules explain oxygen's gaseous nature at room temperature. The constant, random motion of these molecules is what defines the gaseous state.
Oxygen's Role in Respiration and Combustion
Oxygen's gaseous state is essential for its biological function. In respiration, organisms utilize oxygen to break down glucose, releasing energy for cellular processes. This process, called aerobic respiration, is fundamental to the survival of most living things. In combustion, oxygen acts as an oxidizing agent, reacting with fuels to produce energy in the form of heat and light. This is why oxygen is crucial for fire, a process involving rapid oxidation.
Liquid Oxygen: A Cryogenic Fluid
To transition oxygen from its gaseous state to a liquid, we need to significantly lower its temperature. Liquid oxygen (LOX) is produced by cooling gaseous oxygen to its boiling point, which is approximately -183°C (-297°F) at standard atmospheric pressure.
Properties of Liquid Oxygen
Liquid oxygen is a pale blue liquid that is highly reactive. Because of its low temperature, handling LOX requires special safety precautions to prevent frostbite and other hazards associated with cryogenic fluids. It's significantly denser than gaseous oxygen, occupying a much smaller volume for the same mass.
Applications of Liquid Oxygen
The cryogenic properties of LOX make it indispensable in several industrial and scientific applications. It serves as an oxidizer in rocket propellants, providing the necessary oxygen for combustion in space. In medicine, LOX is used in oxygen therapy to supplement breathing for patients with respiratory problems. Furthermore, liquid oxygen finds application in metal fabrication processes involving welding and cutting, providing the necessary oxygen for efficient combustion.
Solid Oxygen: A Pale Blue Crystalline Solid
Further cooling liquid oxygen below its freezing point of -218°C (-360°F) at standard pressure transforms it into a solid. Solid oxygen, like liquid oxygen, is a pale blue color.
The Crystalline Structure of Solid Oxygen
Solid oxygen exhibits different crystalline structures depending on the temperature and pressure. At low pressures, it forms a pale blue, hexagonal close-packed crystal structure. As the pressure increases, the structure transforms into more complex arrangements, exhibiting various magnetic and electronic properties. The detailed analysis of these crystal structures contributes to a deeper understanding of oxygen's behavior at extreme conditions.
Applications of Solid Oxygen
Solid oxygen's applications are less common than liquid oxygen, primarily due to the challenges associated with maintaining its extremely low temperature. However, research involving solid oxygen contributes significantly to our understanding of materials science and the behavior of matter under extreme conditions. Its study is important in fields such as cryogenics, materials science, and astrophysics.
Phase Transitions and Phase Diagrams
The transitions between the solid, liquid, and gaseous states of oxygen are governed by changes in temperature and pressure. These transitions can be visualized using a phase diagram, a graph that illustrates the different phases of a substance as a function of temperature and pressure.
Understanding Oxygen's Phase Diagram
Oxygen's phase diagram shows distinct regions corresponding to the solid, liquid, and gaseous phases. The lines separating these regions represent the conditions under which phase transitions occur. For instance, the line separating the liquid and gas regions indicates the boiling point of oxygen at different pressures. The line separating the solid and liquid regions indicates the melting point, and the line separating the solid and gas regions indicates the sublimation point (transition from solid to gas).
Triple Point and Critical Point
The triple point on the phase diagram represents the unique temperature and pressure at which all three phases (solid, liquid, and gas) coexist in equilibrium. For oxygen, this point lies at a very low temperature and pressure. The critical point indicates the temperature and pressure above which the distinction between liquid and gas disappears, resulting in a supercritical fluid.
The Significance of Oxygen's States
The ability of oxygen to exist in different states plays a crucial role in several areas:
-
Biological processes: The gaseous state of oxygen is fundamental to respiration and the survival of most life forms.
-
Industrial applications: Liquid oxygen is essential for rocket propulsion, welding, and various medical applications.
-
Scientific research: The study of oxygen in its various states contributes to our understanding of materials science, cryogenics, and the behavior of matter under extreme conditions.
-
Environmental science: The concentration of oxygen in the atmosphere plays a vital role in the Earth's climate and ecosystem.
-
Space exploration: Liquid oxygen is a key component of rocket propellants, enabling space travel and exploration.
Safety Precautions when Handling Oxygen
Due to its highly reactive nature, particularly in liquid and solid forms, special safety precautions are necessary when handling oxygen:
-
Avoid flammable materials: Oxygen supports combustion, so keep away from any flammable materials to prevent fire hazards.
-
Proper ventilation: Ensure adequate ventilation in areas where oxygen is used to avoid oxygen enrichment.
-
Cryogenic safety: When working with liquid or solid oxygen, use appropriate cryogenic safety equipment to prevent frostbite and other hazards associated with extremely low temperatures.
-
Pressure regulation: Use pressure regulators and safety valves to control the pressure of gaseous oxygen.
Conclusion: Oxygen's Versatile Nature
In conclusion, oxygen's ability to exist as a solid, liquid, or gas, depending on temperature and pressure, highlights its versatile nature. While we commonly experience it as a life-sustaining gas, understanding its other states and their applications is crucial to appreciate its significance across various scientific disciplines and industrial processes. From supporting life on Earth to powering rockets into space, oxygen's multifaceted behavior continues to shape our world in profound ways. Further research into oxygen's behavior under extreme conditions promises even more fascinating discoveries and potentially transformative applications.
Latest Posts
Latest Posts
-
What Is 20 In Decimal Form
Apr 08, 2025
-
1 Sqrt X 2 1 Integral
Apr 08, 2025
-
What Are The Rungs Of The Ladder Made Of
Apr 08, 2025
-
The Mass Of A Mole Of Nacl Is The
Apr 08, 2025
-
How Many Light Minutes Away Is The Sun
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Is Oxygen A Solid Liquid Or A Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.