Is Hydrogen Chloride Soluble In Water
listenit
Mar 22, 2025 · 6 min read
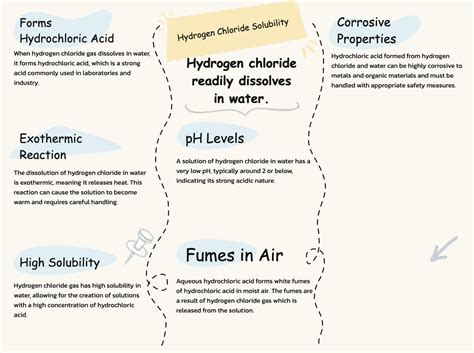
Table of Contents
Is Hydrogen Chloride Soluble in Water? A Deep Dive into the Chemistry
Hydrogen chloride (HCl), a colorless gas with a pungent, irritating odor, exhibits a remarkable property: high solubility in water. This seemingly simple statement belies a rich tapestry of chemical interactions and physical phenomena that are crucial to understanding its behavior and applications. This article will explore the solubility of hydrogen chloride in water, delving into the underlying chemistry, the factors influencing solubility, and the significant consequences of this interaction.
Understanding the Dissolution Process
The solubility of HCl in water is a result of a strong acid-base reaction. When HCl gas comes into contact with water, it undergoes ionization, or dissociation, into its constituent ions: a proton (H⁺) and a chloride ion (Cl⁻). This process can be represented by the following equation:
HCl(g) + H₂O(l) → H₃O⁺(aq) + Cl⁻(aq)
The hydronium ion (H₃O⁺) is formed when the proton from HCl bonds with a water molecule. This reaction is essentially an acid-base reaction where HCl acts as a Brønsted-Lowry acid, donating a proton, and water acts as a Brønsted-Lowry base, accepting the proton. The strength of this acid-base interaction is a primary driver of HCl's high solubility.
The resulting solution is known as hydrochloric acid (HCl(aq)), a strong acid that is widely used in various industrial and laboratory applications. The high concentration of ions in the solution is a direct consequence of the near-complete ionization of HCl in water.
Factors Affecting the Solubility of Hydrogen Chloride
While HCl is highly soluble in water, several factors can influence the extent of its solubility:
1. Temperature:
Solubility generally increases with temperature. However, the relationship between temperature and HCl solubility is more complex than a simple linear increase. While higher temperatures can initially enhance the dissolution rate, it also affects the equilibrium between gaseous HCl and dissolved HCl, potentially leading to some decrease in the overall solubility at extremely high temperatures. This is because the equilibrium shifts slightly towards the gaseous phase at higher temperatures, as the exothermic nature of the dissolution process favors lower temperatures.
2. Pressure:
As HCl is a gas, pressure plays a significant role in its solubility. Henry's Law dictates that the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid. Increasing the partial pressure of HCl above the water surface will force more HCl molecules into the solution, increasing its solubility. This principle is commonly applied in industrial processes to increase the efficiency of HCl dissolution.
3. Polarity:
The high solubility of HCl in water is directly related to the polar nature of both the solute and the solvent. Water is a highly polar molecule due to its bent molecular geometry and the electronegativity difference between oxygen and hydrogen. HCl is also a polar molecule, with the hydrogen atom carrying a partial positive charge and the chlorine atom carrying a partial negative charge. This like-dissolves-like principle ensures strong electrostatic interactions between the polar HCl molecules and the polar water molecules, facilitating dissolution.
4. Intermolecular Forces:
The strong hydrogen bonding in water is crucial for dissolving HCl. When HCl dissolves, the hydrogen atoms of HCl interact favorably with the oxygen atoms of water molecules through hydrogen bonds. Simultaneously, the chloride ions are stabilized by ion-dipole interactions with water molecules. These interactions significantly contribute to the high energy gain upon dissolution, making the process thermodynamically favorable.
Consequences of HCl's High Solubility
The high solubility of HCl in water has several important consequences:
1. Formation of a Strong Acid:
The most significant consequence is the formation of hydrochloric acid, a strong acid with numerous industrial applications. Its high acidity allows for its use in processes requiring strong acidic conditions, such as:
- Metal cleaning: HCl is used to remove oxides and other impurities from metal surfaces.
- Pickling: In the steel industry, HCl is used to remove surface scale from steel.
- Chemical synthesis: HCl serves as a reagent in countless chemical reactions.
2. Environmental Impact:
The high solubility of HCl makes it a potential environmental pollutant. Accidental releases of HCl gas can readily dissolve in atmospheric water vapor, forming acidic rain. This acidic rain can have damaging effects on vegetation, aquatic ecosystems, and infrastructure.
3. Safety Considerations:
Hydrochloric acid is a corrosive substance, and its high solubility in water allows for rapid and extensive damage upon contact with skin or mucous membranes. Appropriate safety precautions, including the use of personal protective equipment (PPE), are crucial when handling HCl solutions.
Comparing HCl Solubility to Other Gases
To further appreciate the high solubility of HCl in water, let's compare it to other common gases:
- Oxygen (O₂): Oxygen is relatively insoluble in water. Its non-polar nature and weak interactions with water molecules limit its solubility.
- Carbon Dioxide (CO₂): Carbon dioxide is moderately soluble in water, forming carbonic acid (H₂CO₃). While it forms an acid, the interaction is weaker than with HCl, resulting in lower solubility.
- Ammonia (NH₃): Ammonia is highly soluble in water, forming ammonium hydroxide (NH₄OH). Similar to HCl, its polar nature and ability to participate in acid-base reactions contribute to its high solubility. However, the resulting solution is a weak base, unlike the strong acid formed from HCl.
Applications of Hydrochloric Acid
The high solubility of hydrogen chloride, leading to the formation of hydrochloric acid, underpins its vast array of applications across diverse industries:
- Food processing: Hydrochloric acid aids in regulating acidity in food products.
- Pharmaceutical industry: It plays a crucial role in the synthesis of various pharmaceuticals.
- Petroleum refining: HCl is involved in the catalytic processes used in oil refining.
- Leather tanning: It's employed in the process of tanning hides.
- Wastewater treatment: Hydrochloric acid is utilized in neutralizing alkaline wastewater.
- Metalworking: In etching, pickling, and cleaning metals.
Conclusion: The Significance of HCl's Solubility
The high solubility of hydrogen chloride in water is a fundamental chemical property with far-reaching consequences. The resulting strong acid, hydrochloric acid, is a cornerstone of numerous industrial processes and plays a critical role in various scientific fields. Understanding the factors that govern HCl's solubility, and the implications of its dissolution, is essential for safe handling, environmentally responsible use, and the continued development of applications based on this remarkable chemical reaction. The interplay between the polar nature of HCl and water, the strength of the acid-base reaction, and the influence of temperature and pressure all combine to make HCl a uniquely soluble gas with profound implications for chemistry and industry. Further research continues to explore the intricacies of this interaction, uncovering new applications and refining our understanding of this fundamental chemical phenomenon.
Latest Posts
Latest Posts
-
Common Multiple Of 8 And 7
Mar 23, 2025
-
How Much Is 30 Liters In Gallons
Mar 23, 2025
-
What Is The Product Of And
Mar 23, 2025
-
What Is 33 Out Of 40 As A Percentage
Mar 23, 2025
-
Why Is Francium The Most Reactive Metal
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Is Hydrogen Chloride Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
