Is Gold Part Of The Transition Metal
listenit
Mar 19, 2025 · 5 min read
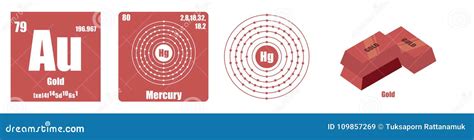
Table of Contents
Is Gold Part of the Transition Metal?
The question of whether gold is a transition metal often sparks debate, even among seasoned chemists. While seemingly straightforward, the classification of elements hinges on a nuanced understanding of their electronic configurations and chemical properties. This comprehensive article will delve into the intricacies of transition metal definition, examine gold's electronic structure and chemical behavior, and ultimately answer the question definitively, while exploring related concepts and controversies.
Understanding Transition Metals: A Definition
Transition metals, located in the d-block of the periodic table (groups 3-12), are characterized by their partially filled d-orbitals in at least one of their oxidation states. This incomplete d-orbital configuration is the cornerstone of their unique chemical properties, notably their variable oxidation states, catalytic activity, and formation of colorful complexes. The presence of these d-electrons allows for the formation of multiple bonds and complex ions, influencing their magnetic properties and reactivity.
Key Characteristics of Transition Metals:
- Variable Oxidation States: Transition metals can exist in multiple oxidation states, meaning they can readily lose different numbers of electrons to form ions. This versatility contributes to their diverse chemistry.
- Formation of Colored Complexes: The d-electrons readily absorb and emit light of specific wavelengths, leading to the formation of brightly colored coordination compounds. The color is highly dependent on the ligand environment and the oxidation state of the metal.
- Catalytic Activity: The partially filled d-orbitals allow transition metals to act as catalysts, facilitating numerous chemical reactions. This is crucial in various industrial processes and biological systems.
- Paramagnetism: Many transition metal ions possess unpaired electrons in their d-orbitals, leading to paramagnetism—a weak attraction to magnetic fields.
- High Melting and Boiling Points: The strong metallic bonding arising from the interaction of d-electrons contributes to their high melting and boiling points.
- Formation of Alloys: Transition metals readily form alloys with other metals, resulting in materials with enhanced properties, such as strength and corrosion resistance.
Gold's Electronic Configuration and Oxidation States
Gold (Au), element 79, possesses the electronic configuration [Xe] 4f<sup>14</sup> 5d<sup>10</sup> 6s<sup>1</sup>. This configuration initially seems to contradict the definition of a transition metal, as its d-orbital is completely filled. However, the story isn't that simple.
The seemingly full d<sup>10</sup> configuration doesn't fully encompass the complexity of gold's behavior. While in its ground state the d-orbital is filled, the readily accessible higher energy levels mean that gold can participate in reactions where electrons from the 6s and even 5d orbitals are involved. This allows for the formation of several oxidation states, including +1 and +3, which are the most common. The +1 state is particularly interesting because it involves the oxidation of a single 6s electron, highlighting the unique behavior of gold's electron configuration.
Gold's Oxidation States and their Implications:
- +1 (Au<sup>+</sup>): This oxidation state is relatively stable and seen in compounds like gold(I) chloride (AuCl). The stability of the +1 oxidation state is less common for transition metals.
- +3 (Au<sup>3+</sup>): This is another common oxidation state, exemplified in gold(III) chloride (AuCl<sub>3</sub>). The formation of the +3 state involves the loss of an electron from the 5d orbital, showcasing the involvement of d-electrons in the oxidation process.
- Other Oxidation States: While less common, gold can exhibit other, less stable oxidation states such as +2 and +5, but these are usually short-lived and exist under specific conditions.
The ability to exhibit variable oxidation states, even if not as extensive as some other transition metals, aligns with a key characteristic of the transition metals. This demonstrates that the definition of a transition metal is not merely about a partially filled d-orbital in the ground state.
Gold's Chemical Behavior: Supporting its Transition Metal Status
Beyond its electronic structure, gold's chemical behavior further strengthens its classification as a transition metal. Let’s explore some key aspects:
- Complex Formation: Gold forms numerous coordination complexes with various ligands, exhibiting characteristic behavior of transition metals. The coordination complexes of gold display a rich variety of colors and structures, influencing their applications in catalysis and medicine.
- Catalytic Properties: Although not as widely used as some other transition metals like platinum or palladium, gold displays catalytic activity in certain reactions, especially at the nanoscale. Gold nanoparticles, in particular, have shown promise in catalysis, highlighting the involvement of d-electrons in their reactivity.
- Alloy Formation: Gold readily forms alloys with other metals, a property common among transition metals. These alloys exhibit modified properties, such as improved hardness, ductility, and corrosion resistance. This makes them crucial in jewelry and electronic applications.
- Relativistic Effects: Gold's position in the periodic table leads to relativistic effects influencing its properties. These effects significantly impact its electronic structure and chemical behavior, altering its reactivity and bond lengths. Relativistic effects, while unique, don’t negate its overall transition metal characteristics.
Addressing the Controversies and Nuances
While the evidence strongly suggests gold is a transition metal, some arguments exist against this classification. The main point of contention is the completely filled d<sup>10</sup> orbital in its ground state. However, this is a simplistic view.
The dynamic nature of electronic configurations, particularly considering readily available higher energy orbitals and the involvement of d-electrons in oxidation and complex formation, surpasses the limitations of the static ground-state configuration. The definition should encompass the oxidation states and chemical behavior exhibited throughout its various reactions. Focusing solely on the ground state electron configuration neglects the crucial aspect of its chemical reactivity.
Conclusion: Gold is a Transition Metal
In conclusion, despite its completely filled d-orbital in the ground state, gold is unequivocally a transition metal. Its variable oxidation states (+1, +3, and others), its ability to form a vast array of coordination complexes, its catalytic properties (especially in nanoparticle form), its alloying behavior, and the influence of relativistic effects all align with the defining characteristics of transition metals. The dynamic nature of electron configuration and the importance of higher energy level participation in chemical reactions must be considered for an accurate classification. While nuances and debates exist, the overwhelming evidence firmly places gold within the transition metal family. Understanding the broader context of electronic behavior and chemical reactivity is crucial for a correct and complete classification of the elements.
Latest Posts
Latest Posts
-
2000 Mg Is Equal To How Many G
Mar 20, 2025
-
What Element Has The Lowest Electronegativity
Mar 20, 2025
-
Which Layer Of The Atmosphere Does Weather Occur In
Mar 20, 2025
-
The Si Unit For Power Is The
Mar 20, 2025
-
How Much Is 1 4 Lb
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Is Gold Part Of The Transition Metal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
