Is Chlorine A Metal Or Nonmetal
listenit
Mar 15, 2025 · 5 min read
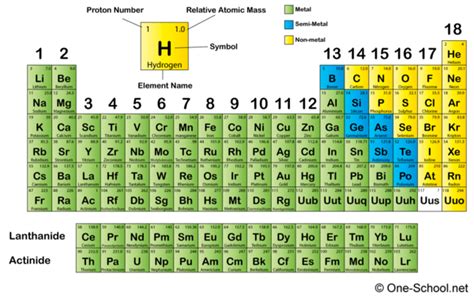
Table of Contents
Is Chlorine a Metal or Nonmetal? A Deep Dive into its Properties and Reactivity
Chlorine, a ubiquitous element found in everyday life, often sparks curiosity regarding its classification. Is it a metal or a nonmetal? The answer, unequivocally, is nonmetal. But understanding why it's a nonmetal requires a deeper exploration of its atomic structure, chemical behavior, and physical properties. This article will delve into these aspects, clarifying chlorine's classification and highlighting its importance in various contexts.
Understanding the Metal vs. Nonmetal Dichotomy
Before we pinpoint chlorine's position, let's establish the fundamental differences between metals and nonmetals. This distinction isn't solely based on visual appearance but primarily stems from their electronic configurations and the resultant chemical properties.
Metals: A Shared Characteristic
Metals are generally characterized by their:
- Excellent electrical and thermal conductivity: Electrons in metals are delocalized, meaning they're not tightly bound to individual atoms, allowing for easy electron flow. This explains their ability to conduct electricity and heat efficiently.
- Malleability and ductility: Metals can be hammered into thin sheets (malleability) and drawn into wires (ductility) without breaking, due to the "sea" of delocalized electrons that allow atoms to slide past each other.
- Metallic luster: The interaction of light with the delocalized electrons gives metals their characteristic shiny appearance.
- High melting and boiling points: The strong metallic bonds require significant energy to overcome, resulting in high melting and boiling points (with exceptions).
- Tendency to lose electrons: Metals readily lose electrons to form positively charged ions (cations). This is because they have relatively low ionization energies.
Nonmetals: A Contrasting Profile
Nonmetals, conversely, exhibit:
- Poor electrical and thermal conductivity: Electrons in nonmetals are tightly bound to individual atoms, hindering electron movement and resulting in poor conductivity.
- Brittleness: Nonmetals tend to be brittle and shatter easily when subjected to stress.
- Lack of metallic luster: Nonmetals generally lack the shiny appearance of metals.
- Low melting and boiling points (generally): The weaker interatomic forces in nonmetals require less energy to break, leading to lower melting and boiling points (with exceptions).
- Tendency to gain electrons: Nonmetals readily gain electrons to form negatively charged ions (anions), as they have high electron affinities.
Chlorine: A Definitive Nonmetal
Chlorine, with its atomic number 17 and electronic configuration [Ne]3s²3p⁵, clearly fits the profile of a nonmetal. Let's examine its properties:
Physical Properties of Chlorine
- State at room temperature: Chlorine exists as a yellowish-green gas at room temperature and standard pressure. This is atypical of metals, which are usually solids at room temperature.
- Melting and Boiling Points: Chlorine has relatively low melting (-101.5 °C) and boiling points (-34.0 °C), consistent with nonmetals.
- Density: It's denser than air.
- Conductivity: Chlorine is a poor conductor of electricity and heat, confirming its nonmetallic nature.
- Appearance: Its yellowish-green color is far from the metallic luster commonly observed in metals.
- Brittleness (in solid state): Solid chlorine is brittle, a characteristic often found in nonmetals.
Chemical Properties of Chlorine
- Electronegativity: Chlorine has a high electronegativity, meaning it strongly attracts electrons in chemical bonds. This is a hallmark of nonmetals.
- Oxidation States: Chlorine exhibits various oxidation states, predominantly -1, +1, +3, +5, and +7. This versatility is common in nonmetals, reflecting their ability to gain or share electrons in different ways.
- Reactivity: Chlorine is highly reactive, readily forming compounds with many other elements. Its reactivity stems from its strong tendency to gain an electron to achieve a stable octet configuration. This high reactivity is a characteristic of many nonmetals.
- Formation of Covalent Bonds: Chlorine predominantly forms covalent bonds, sharing electrons with other atoms to achieve a stable electron configuration. This is a typical characteristic of nonmetals, contrasting with the ionic bonds commonly found in metal compounds.
- Formation of Acids: Chlorine readily forms acids such as hydrochloric acid (HCl), showcasing another typical characteristic of nonmetals.
Chlorine's Role in Various Applications
Chlorine's unique properties make it indispensable in various applications:
Water Treatment: Essential for Public Health
Chlorine plays a crucial role in water purification and disinfection. It effectively eliminates harmful bacteria, viruses, and other microorganisms, safeguarding public health. The addition of chlorine to water forms hypochlorous acid, a potent disinfectant. Understanding chlorine's nonmetallic properties—particularly its high reactivity and ability to readily oxidize organic compounds—is key to its effectiveness in this process.
Industrial Applications: Diverse and Wide-Ranging
Chlorine is a vital industrial chemical, contributing to the manufacturing of countless products:
- Plastics (PVC): Chlorine is a key component in the production of polyvinyl chloride (PVC), a versatile plastic widely used in pipes, flooring, and various other applications.
- Solvents: Chlorine-containing compounds find use as solvents in various industrial processes.
- Bleaches: Chlorine-based compounds are effective bleaching agents used in paper and textile industries.
- Pharmaceuticals: Chlorine is present in many pharmaceuticals, contributing to their effectiveness and properties.
- Pesticides: Certain chlorine-containing compounds are used as pesticides.
Understanding the Risks: Handling Chlorine Safely
While essential, chlorine can pose risks if not handled appropriately. It's a highly reactive element and its gas form can be toxic, even lethal at high concentrations. Therefore, appropriate safety measures are crucial during its handling, storage, and usage. Understanding its chemical properties, specifically its reactivity and potential for forming harmful compounds, is vital for safe practices.
Debunking Common Misconceptions
Several misconceptions surrounding chlorine's classification need addressing:
- Visual appearance: Some might mistakenly associate chlorine's yellowish-green color with metals, but color is not a definitive indicator of metallic or nonmetallic character.
- Reactivity: High reactivity isn't unique to metals; many nonmetals are also highly reactive. Chlorine's reactivity is a direct consequence of its electronic structure and its strong tendency to gain an electron.
- Conductivity: While some metals are excellent conductors, the lack of conductivity is a more definitive indicator of nonmetallic behavior.
Conclusion: Chlorine's Unmistakable Nonmetal Status
The evidence overwhelmingly confirms that chlorine is a nonmetal. Its physical and chemical properties align perfectly with the characteristics of nonmetals: poor conductivity, low melting and boiling points, high electronegativity, a tendency to gain electrons, and the formation of covalent bonds. Its significance in diverse applications further underscores the importance of understanding its nonmetallic behavior and its crucial role in various aspects of modern life. From purifying our drinking water to forming the basis of numerous industrial materials, chlorine's unique properties, firmly rooted in its nonmetallic classification, continue to shape our world. Further study of its reactivity and compound formation remains vital for responsible and safe utilization of this significant element.
Latest Posts
Latest Posts
-
64 Oz Equals How Many Pounds
Mar 15, 2025
-
How Are Limiting Factors Related To Carrying Capacity
Mar 15, 2025
-
What Percent Is 24 Of 96
Mar 15, 2025
-
8 Of 40 Is What Percent
Mar 15, 2025
-
How Many Obtuse Angles Are In An Obtuse Triangle
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Is Chlorine A Metal Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
