Is Boil A Physical Or Chemical Change
listenit
Mar 15, 2025 · 5 min read
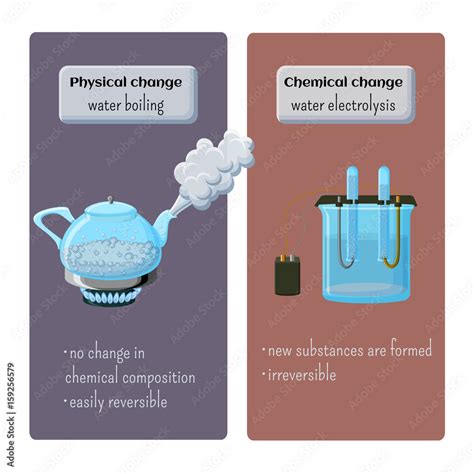
Table of Contents
Is Boiling a Physical or Chemical Change? A Deep Dive into Phase Transitions
The question of whether boiling is a physical or chemical change is a fundamental one in science, often cropping up in introductory chemistry and physics classes. While seemingly simple, the answer requires a nuanced understanding of the differences between physical and chemical changes and the nature of phase transitions. This comprehensive article will delve deep into the process of boiling, examining its characteristics and providing a definitive answer, supported by scientific evidence.
Understanding Physical and Chemical Changes
Before tackling the boiling question, let's establish a clear definition of physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties—like shape, size, or state—are modified. Examples include:
- Melting ice: Ice (solid water) turns into liquid water, but the chemical formula (H₂O) remains unchanged.
- Crushing a can: The can's shape changes, but it's still made of the same metal.
- Dissolving sugar in water: The sugar disappears into the water, but it's still sugar; you can recover it by evaporating the water.
Key characteristics of physical changes include:
- No new substance is formed.
- Changes are usually reversible. For example, liquid water can be frozen back into ice.
- Changes involve a transfer of energy, but not a rearrangement of atoms.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the alteration of the chemical composition of a substance. New substances with different properties are formed. Examples include:
- Burning wood: Wood reacts with oxygen to produce ashes, carbon dioxide, and water—completely different substances.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a new compound with different properties.
- Baking a cake: The ingredients undergo chemical reactions to create a new substance—the cake—with different properties than the individual ingredients.
Key characteristics of chemical changes include:
- New substances are formed with different properties.
- Changes are usually irreversible. You can't easily turn ashes back into wood.
- Changes involve the rearrangement of atoms and the breaking and forming of chemical bonds.
Analyzing the Boiling Process
Now, let's scrutinize the boiling process to determine its classification. Boiling is the rapid vaporization of a liquid, which occurs when a liquid is heated to its boiling point. At this point, the liquid's vapor pressure equals the atmospheric pressure. This allows bubbles of vapor to form within the liquid and rise to the surface.
Let's consider water as an example. When water boils, it transitions from a liquid state to a gaseous state (steam). The chemical formula remains H₂O. No new substance is created. The molecules are simply moving faster and further apart, transitioning from a close-packed liquid arrangement to a more dispersed gaseous arrangement.
Key observations regarding boiling:
- No new chemical substance is formed: The chemical composition of the water remains unchanged; it's still H₂O.
- The change is reversible: The steam can be condensed back into liquid water.
- Energy is absorbed: Heat energy is required to increase the kinetic energy of the water molecules, allowing them to overcome intermolecular forces and transition to the gaseous phase. This is a physical change characteristic.
Boiling: A Physical Change
Based on the above analysis, we can definitively conclude that boiling is a physical change. The process involves a change of state—from liquid to gas—without altering the chemical composition of the substance. While energy is involved, it's used to overcome intermolecular forces rather than breaking chemical bonds. The reversibility of the process further strengthens this conclusion.
Exploring Related Concepts: Evaporation vs. Boiling
It's crucial to differentiate boiling from evaporation. Both involve the transition of a liquid to a gas, but they differ in how this transition occurs:
- Boiling: Occurs throughout the liquid at the boiling point, forming bubbles of vapor within the liquid. It's a rapid and vigorous process.
- Evaporation: Occurs only at the surface of a liquid at any temperature below the boiling point. It's a slower process.
Both evaporation and boiling are physical changes, as they do not involve altering the chemical composition of the substance. However, boiling is a more energetic and rapid phase transition than evaporation.
The Role of Pressure and Temperature in Boiling
The boiling point of a liquid is not a fixed value; it's dependent on the surrounding pressure. At higher altitudes, where atmospheric pressure is lower, the boiling point of water is lower. Conversely, at higher pressures, the boiling point increases. This illustrates how external factors influence the physical change of boiling but do not alter the inherent chemical nature of the substance.
Misconceptions about Boiling
Some might mistakenly consider boiling a chemical change due to the observation of bubbles. These bubbles are not the result of a new substance forming, but rather vaporized liquid escaping the surface. The visual effect of bubbling does not inherently signify a chemical reaction.
Conclusion: A Clear and Definitive Answer
The evidence overwhelmingly supports the conclusion that boiling is a physical change, not a chemical change. It's a phase transition that involves a change in state (liquid to gas) without any alteration to the chemical composition of the substance. The process is reversible, and no new substances are formed. Understanding this distinction is crucial for grasping the fundamentals of physical science and chemical reactions. The role of temperature and pressure further clarifies that the boiling point itself is a physical property influenced by external factors, but it doesn't fundamentally change the nature of the boiling process as a physical transformation. Therefore, while seemingly simple, the question of whether boiling is a physical or chemical change illustrates the complexity and nuance needed to analyze scientific phenomena.
Latest Posts
Latest Posts
-
The Density Of Ethanol Is 0 789 G Ml
May 09, 2025
-
Find The Length Of The Polar Curve
May 09, 2025
-
How Are Lactic And Alcoholic Fermentation Similar
May 09, 2025
-
How Do You Find The Side Length Of A Square
May 09, 2025
-
Will Crayons Melt In The Car
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Boil A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.