Is Air A Element Compound Or Mixture
listenit
Mar 19, 2025 · 5 min read
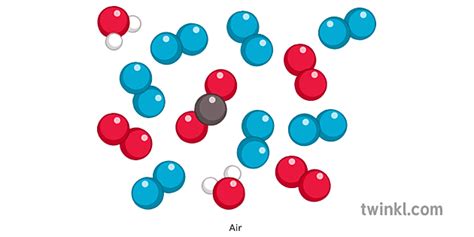
Table of Contents
Is Air an Element, Compound, or Mixture? A Deep Dive into Atmospheric Composition
The question of whether air is an element, compound, or mixture is a fundamental one in chemistry and atmospheric science. The simple answer is that air is a mixture. However, understanding why it's a mixture and the complexities of its composition requires a deeper exploration. This article will delve into the specifics, examining the different components of air, their properties, and how they interact to create the atmosphere we breathe.
Understanding the Definitions: Element, Compound, and Mixture
Before we analyze the composition of air, let's establish clear definitions for the three classifications:
-
Element: An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. Elements cannot be broken down into simpler substances by chemical means. Examples include oxygen (O), nitrogen (N), and hydrogen (H).
-
Compound: A compound is a pure substance formed when two or more different chemical elements are chemically bonded together. These bonds create a new substance with properties distinct from its constituent elements. Water (H₂O) is a classic example; it's a compound made of hydrogen and oxygen atoms. Compounds can be broken down into simpler substances through chemical reactions.
-
Mixture: A mixture is a combination of two or more substances that are not chemically bonded. The substances retain their individual chemical properties and can be separated by physical means like filtration, distillation, or evaporation. Air, as we will see, perfectly exemplifies a mixture.
The Composition of Air: A Mixture of Gases
Air is primarily a gaseous mixture, meaning it's a combination of various gases, each retaining its individual properties. The main components are:
1. Nitrogen (N₂): The Dominant Component
Nitrogen constitutes approximately 78% of Earth's atmosphere by volume. It's a relatively inert gas, meaning it doesn't readily react with other substances. While essential for life, plants and animals cannot directly utilize atmospheric nitrogen. Instead, specialized bacteria convert it into usable forms through a process called nitrogen fixation.
2. Oxygen (O₂): Essential for Life
Oxygen makes up roughly 21% of the atmosphere. It's a highly reactive gas, crucial for respiration in most living organisms. Oxygen plays a critical role in combustion and many other chemical processes.
3. Argon (Ar): An Inert Noble Gas
Argon accounts for approximately 0.93% of the atmosphere. It's a noble gas, meaning it's chemically inert and rarely participates in reactions. Argon is often used in industrial applications, such as welding and lighting.
4. Trace Gases: Important in Small Amounts
Besides the major components, air also contains several trace gases present in much smaller quantities but with significant impacts on the environment and life:
-
Carbon Dioxide (CO₂): A vital greenhouse gas, essential for photosynthesis in plants. Human activities, especially the burning of fossil fuels, have significantly increased atmospheric CO₂, contributing to climate change.
-
Neon (Ne), Helium (He), Methane (CH₄), Krypton (Kr), Hydrogen (H₂), and Xenon (Xe): These gases are present in trace amounts and exhibit varied properties. Some, like methane, are potent greenhouse gases, while others, like helium, are used in various technological applications.
-
Water Vapor (H₂O): The amount of water vapor in the air varies significantly depending on location and weather conditions. It's an essential component of the water cycle and plays a crucial role in regulating Earth's temperature.
-
Ozone (O₃): Ozone in the stratosphere forms a protective layer that absorbs harmful ultraviolet (UV) radiation from the sun. However, ground-level ozone is a pollutant, contributing to respiratory problems.
Why Air is a Mixture, Not a Compound
Several key factors confirm that air is a mixture and not a compound:
-
Variable Composition: The proportions of gases in air vary depending on location, altitude, and weather conditions. A compound has a fixed and definite composition, unlike air.
-
No Chemical Bonds: The gases in air are not chemically bonded to one another. They exist independently and retain their individual properties. In contrast, a compound has its constituent elements chemically linked through bonds.
-
Separation by Physical Means: The components of air can be separated by physical methods like fractional distillation of liquid air. This process separates the gases based on their boiling points, a characteristic impossible for a compound. A compound requires chemical reactions to be broken down into its elements.
-
Retention of Individual Properties: Each gas in air maintains its individual chemical and physical properties. This is different from a compound, where the properties of the resulting substance are often distinct from its constituent elements.
The Importance of Understanding Air's Composition
Understanding the composition of air is vital for several reasons:
-
Environmental Monitoring: Tracking changes in air composition, especially greenhouse gases and pollutants, is crucial for monitoring environmental health and mitigating climate change.
-
Medical Applications: The concentration of gases like oxygen is vital in medical settings, impacting respiratory care and treatment.
-
Industrial Processes: Various industries rely on the properties of specific gases present in air, impacting manufacturing, production, and safety protocols.
-
Atmospheric Research: Studying air composition helps researchers understand atmospheric processes, weather patterns, and the impact of human activities on the planet.
Air Pollution: Altering the Natural Balance
Human activities have significantly altered the composition of air, primarily through the emission of pollutants. These pollutants can have detrimental effects on human health and the environment:
-
Greenhouse Gases: Increased levels of CO₂, methane, and other greenhouse gases trap heat in the atmosphere, leading to global warming and climate change.
-
Airborne Particulates: Tiny particles from combustion and industrial processes contribute to respiratory problems and can have a significant impact on air quality.
-
Acid Rain: Emissions of sulfur dioxide and nitrogen oxides contribute to acid rain, damaging ecosystems and infrastructure.
-
Ozone Depletion: Certain chemicals, such as chlorofluorocarbons (CFCs), have depleted the ozone layer in the stratosphere, increasing exposure to harmful UV radiation.
Conclusion: Air – A Dynamic and Vital Mixture
In conclusion, air is definitively a mixture of gases, not a compound or an element. Its composition is complex and dynamic, varying depending on location and environmental factors. Understanding the intricate interplay of its various components is essential for environmental monitoring, public health, and advancing our knowledge of atmospheric science. The ongoing impact of human activities on air quality necessitates continuous monitoring and research to protect both the environment and human health. The seemingly simple question of "What is air?" opens a vast and fascinating world of scientific inquiry.
Latest Posts
Latest Posts
-
1 Or 3 Nitrogen Bases Equal One Amino Acid
May 09, 2025
-
Write The Standard Form Of The Equation Of Each Line
May 09, 2025
-
Find The Value Of Each Determinant
May 09, 2025
-
Does Pf5 Obey The Octet Rule
May 09, 2025
-
An Electron Moving At Constant Speed Produces
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Air A Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.