Identify The Two Key Factors That Determine Nuclear Stability
listenit
Apr 06, 2025 · 6 min read
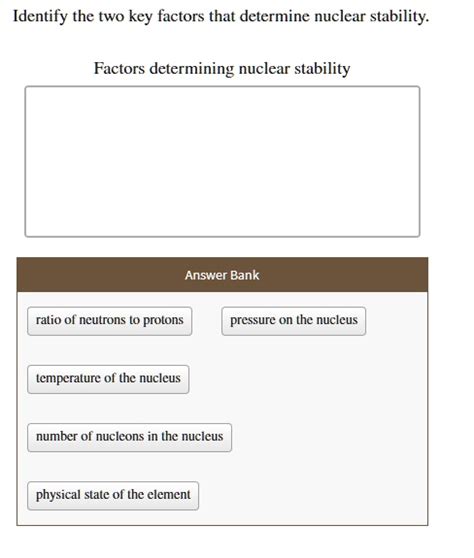
Table of Contents
Identifying the Two Key Factors that Determine Nuclear Stability
Nuclear stability, a cornerstone of nuclear physics, dictates whether an atomic nucleus will remain intact or undergo radioactive decay. Understanding what governs this stability is crucial for various applications, from nuclear power generation to medical treatments and even astrophysics. While the intricacies of nuclear forces are complex, two key factors emerge as paramount in determining nuclear stability: the neutron-to-proton ratio (N/Z ratio) and the number of nucleons (protons and neutrons). This article delves deep into each factor, exploring their influence and exceptions to the rules, providing a comprehensive understanding of nuclear stability.
The Neutron-to-Proton Ratio (N/Z Ratio)
The neutron-to-proton ratio, often represented as N/Z, is a fundamental indicator of nuclear stability. It reflects the balance between the strong nuclear force, which holds protons and neutrons together, and the electromagnetic force, which causes protons to repel each other. Protons, carrying a positive charge, experience a repulsive Coulomb force, while neutrons, being electrically neutral, don't contribute to this repulsion.
The Significance of the Strong Nuclear Force
The strong nuclear force is the dominant force at short ranges within the nucleus, overwhelmingly powerful compared to the electromagnetic force. However, its influence is limited to a very short distance. As the number of protons increases, the cumulative effect of the electromagnetic repulsion grows significantly, threatening to overcome the strong nuclear force’s binding effect. This is where neutrons play a crucial role.
Neutrons as Nuclear Glue
Neutrons enhance nuclear stability by contributing to the strong nuclear force without adding to the electromagnetic repulsion. They act as a kind of "nuclear glue," increasing the overall binding energy of the nucleus and counteracting the repulsive forces between protons. For lighter nuclei (with atomic number Z ≤ 20), a N/Z ratio close to 1 (or even slightly less than 1) generally indicates stability. However, as the atomic number increases, more neutrons are needed to offset the escalating electromagnetic repulsion.
The N/Z Ratio and the "Valley of Stability"
This relationship between N/Z ratio and stability is beautifully visualized in a chart called the "seabed" or "valley of stability". This chart plots the number of neutrons (N) against the number of protons (Z) for known isotopes. Stable isotopes generally cluster around a line representing an optimal N/Z ratio, forming the "valley". Isotopes lying far from this valley are generally unstable and radioactive.
Deviation from the Ideal N/Z Ratio
Deviations from the ideal N/Z ratio result in different types of radioactive decay.
-
Neutron-rich nuclei (N/Z > 1): These nuclei have an excess of neutrons and tend to undergo beta-minus (β⁻) decay. In β⁻ decay, a neutron transforms into a proton, an electron (β⁻ particle), and an antineutrino. This process reduces the number of neutrons and increases the number of protons, moving the nucleus closer to the valley of stability.
-
Proton-rich nuclei (N/Z < 1): These nuclei have a deficiency of neutrons and tend to undergo either positron emission (β⁺ decay) or electron capture. In β⁺ decay, a proton transforms into a neutron, a positron (β⁺ particle), and a neutrino. Electron capture involves a proton capturing an inner electron from the atom, transforming into a neutron and releasing a neutrino. Both processes decrease the number of protons and increase the number of neutrons, again moving the nucleus towards the valley of stability.
-
Nuclei with very high atomic numbers: Even with a favorable N/Z ratio, extremely heavy nuclei become unstable due to the overwhelming influence of the electromagnetic repulsive force. They tend to undergo alpha decay, emitting an alpha particle (a helium nucleus consisting of two protons and two neutrons). This reduces the atomic number and moves the nucleus closer to the valley of stability.
The Number of Nucleons (Magic Numbers)
The sheer number of nucleons – protons and neutrons – also significantly impacts nuclear stability. Certain "magic numbers" of protons or neutrons (2, 8, 20, 28, 50, 82, 126) confer exceptional stability. Nuclei with magic numbers of both protons and neutrons (doubly magic nuclei) are particularly stable.
The Shell Model and Nuclear Stability
This phenomenon is explained by the nuclear shell model, which proposes that nucleons occupy discrete energy levels or shells within the nucleus, similar to electrons in an atom. When a shell is completely filled, the nucleus achieves extra stability, analogous to the stability of noble gases with their full electron shells. Magic numbers correspond to these completely filled shells. Nuclei with these magic numbers exhibit higher binding energies and are less likely to undergo radioactive decay.
Examples of Magic Number Nuclei
Helium-4 (²He), with 2 protons and 2 neutrons, is a classic example of a doubly magic nucleus and is extraordinarily stable. Other examples include ¹⁶O (oxygen-16), ⁴⁰Ca (calcium-40), and ²⁰⁸Pb (lead-208). These nuclei demonstrate exceptional stability compared to their neighbors on the nuclear chart.
Exceptions to the Rules
While the N/Z ratio and magic numbers provide a strong framework for understanding nuclear stability, there are always exceptions. The interplay between the strong nuclear force and the electromagnetic force is incredibly complex, influenced by subtle factors not entirely captured by these simple rules.
Isobaric Analog States
For instance, isobaric analog states represent a slight deviation where nuclei with the same mass number but different proton and neutron numbers exhibit unexpected similarities in energy levels. These states, while technically different isotopes, display a degree of "stability mirroring," influencing decay pathways in ways not fully predicted by the N/Z ratio alone.
Fine Structure of Energy Levels
Furthermore, the fine structure of nuclear energy levels influences the overall stability. Small variations in energy levels can impact the likelihood of different decay modes, leading to subtle deviations from what the N/Z ratio and magic numbers alone would predict.
The Island of Stability
The search for the "island of stability" is a compelling example of the complexities in nuclear stability. Theoretical predictions suggest that superheavy nuclei, far beyond the currently known elements, could possess enhanced stability due to specific combinations of protons and neutrons. The discovery of these superheavy elements would further refine our understanding of the underlying forces governing nuclear structure.
Conclusion
In summary, the stability of an atomic nucleus is a delicate balance determined primarily by two key factors: the neutron-to-proton ratio (N/Z ratio) and the number of nucleons (especially the "magic numbers"). The N/Z ratio reflects the competition between the strong nuclear force and the electromagnetic repulsion, while magic numbers represent the enhanced stability associated with completely filled nuclear shells. While these factors provide a robust framework for understanding nuclear stability, the reality is more nuanced, with subtle interactions and exceptions that continually challenge and refine our understanding of this fundamental aspect of nuclear physics. Continued research in nuclear physics promises to further illuminate the intricate interplay of forces that govern the stability of the atomic nucleus. Exploring this realm helps us not only understand the building blocks of matter but also to harness the power of nuclear reactions for the betterment of humanity.
Latest Posts
Latest Posts
-
Why Is Cosx An Even Function
Apr 07, 2025
-
The Process Of Removing Salt From Seawater Is Called
Apr 07, 2025
-
Y 2x 3 Y 3x 2
Apr 07, 2025
-
Is Condensation Physical Or Chemical Change
Apr 07, 2025
-
Saturn Average Distance From The Sun
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about Identify The Two Key Factors That Determine Nuclear Stability . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
