How To Tell If A Reaction Is Spontaneous
listenit
Mar 16, 2025 · 6 min read
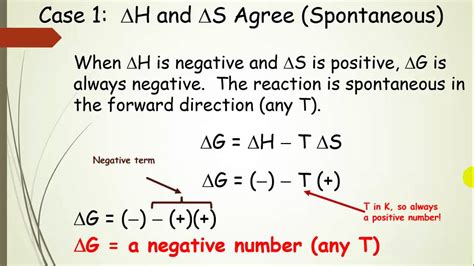
Table of Contents
How to Tell if a Reaction is Spontaneous: A Comprehensive Guide
Determining whether a chemical reaction will occur spontaneously is a fundamental concept in chemistry with far-reaching implications in various fields, from materials science to biology. Spontaneity doesn't necessarily mean a reaction will happen quickly; it simply indicates whether it's thermodynamically favored to proceed under a given set of conditions. This article will delve into the various ways we can predict the spontaneity of a reaction, focusing on both theoretical principles and practical applications.
Understanding Spontaneity: Entropy and Gibbs Free Energy
At the heart of predicting spontaneity lies the concept of Gibbs Free Energy (ΔG). This thermodynamic potential measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. The key relationship is:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content)
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy (disorder)
A negative ΔG indicates a spontaneous reaction, while a positive ΔG indicates a non-spontaneous reaction. A ΔG of zero signifies the reaction is at equilibrium.
Enthalpy (ΔH): The Heat Factor
Enthalpy represents the heat absorbed or released during a reaction.
-
Exothermic reactions (ΔH < 0): These reactions release heat into the surroundings, often feeling warm to the touch. Exothermic reactions are generally favored because they lower the system's energy.
-
Endothermic reactions (ΔH > 0): These reactions absorb heat from the surroundings, often feeling cold. Endothermic reactions require energy input to proceed.
Entropy (ΔS): The Disorder Factor
Entropy is a measure of disorder or randomness within a system. Reactions tend to favor an increase in entropy.
-
Increased entropy (ΔS > 0): This often occurs when the number of gaseous molecules increases, solids dissolve in liquids, or a solid breaks down into smaller components. Increased disorder is statistically more likely.
-
Decreased entropy (ΔS < 0): This happens when the number of gaseous molecules decreases, liquids solidify, or simpler molecules combine to form more complex ones. These reactions are entropically unfavorable.
The interplay of ΔH and ΔS
The spontaneity of a reaction isn't solely determined by enthalpy or entropy alone; it's a delicate balance between the two, as dictated by the Gibbs Free Energy equation. Let's explore the four possible scenarios:
1. ΔH < 0 and ΔS > 0: This is the most favorable scenario. The reaction is both exothermic (releases heat) and increases disorder. ΔG will always be negative, making the reaction spontaneous at all temperatures.
2. ΔH > 0 and ΔS > 0: This scenario involves an endothermic reaction that increases disorder. The spontaneity depends on the temperature. At low temperatures, the TΔS term is small, and ΔG will be positive (non-spontaneous). At high temperatures, the TΔS term becomes significant, eventually overcoming the positive ΔH, leading to a negative ΔG and spontaneous reaction.
3. ΔH < 0 and ΔS < 0: This involves an exothermic reaction that decreases disorder. Similar to the previous case, spontaneity depends on temperature. At low temperatures, the negative TΔS term is small, and ΔG will be negative (spontaneous). At high temperatures, the negative TΔS term becomes large enough to make ΔG positive (non-spontaneous).
4. ΔH > 0 and ΔS < 0: This is the least favorable scenario. The reaction is endothermic and decreases disorder. ΔG will always be positive, making the reaction non-spontaneous at all temperatures.
Practical Methods for Determining Spontaneity
While understanding the Gibbs Free Energy equation is crucial, predicting spontaneity in practice often involves other techniques and considerations:
1. Standard Free Energy Change (ΔG°)
Standard free energy change refers to the change in Gibbs Free Energy under standard conditions (298 K and 1 atm pressure). Tables of standard free energy changes of formation (ΔG°f) for various substances are readily available. The standard free energy change for a reaction can be calculated using:
ΔG° = Σ ΔG°f(products) - Σ ΔG°f(reactants)
A negative ΔG° indicates the reaction is spontaneous under standard conditions.
2. Equilibrium Constant (K)
The equilibrium constant (K) is a measure of the relative amounts of reactants and products at equilibrium. It's related to the standard free energy change by:
ΔG° = -RTlnK
where R is the ideal gas constant and T is the temperature in Kelvin. A large K value (K >> 1) indicates that the equilibrium lies far to the right (favoring products), implying a spontaneous reaction. Conversely, a small K value (K << 1) suggests a non-spontaneous reaction.
3. Electrochemical Cells
For redox reactions, spontaneity can be assessed using electrochemical cells. The cell potential (E°cell) is related to the standard free energy change by:
ΔG° = -nFE°cell
where n is the number of moles of electrons transferred and F is the Faraday constant. A positive E°cell indicates a spontaneous reaction (galvanic cell), while a negative E°cell suggests a non-spontaneous reaction (electrolytic cell).
4. Qualitative Observations
Sometimes, direct observation can provide clues about spontaneity. For instance, if a reaction proceeds readily at room temperature with a visible change (e.g., gas evolution, precipitate formation, color change), it's likely spontaneous. However, this method is highly qualitative and shouldn't be relied upon for precise predictions.
Factors Influencing Spontaneity Beyond Thermodynamics
While thermodynamics dictates whether a reaction is possible, it doesn't dictate how fast it will occur. The rate of a reaction depends on kinetics, which involve factors such as:
-
Activation energy: Even spontaneous reactions may require an initial energy input to overcome the activation energy barrier.
-
Reaction mechanisms: The specific steps involved in a reaction influence its rate.
-
Presence of catalysts: Catalysts can significantly increase reaction rates by lowering the activation energy without affecting the overall ΔG.
Applications and Examples
The ability to predict spontaneity is crucial in numerous fields:
-
Materials Science: Designing materials with desired properties often involves understanding the thermodynamic feasibility of forming specific compounds or structures.
-
Biochemistry: Metabolic pathways rely on spontaneous reactions to drive biological processes. Understanding spontaneity is essential for comprehending energy transfer in living systems.
-
Environmental Science: Predicting the spontaneous formation of pollutants or the degradation of environmental contaminants helps in designing remediation strategies.
-
Industrial Chemistry: Optimizing industrial processes often involves identifying spontaneous reactions that can proceed efficiently under specific conditions.
Examples:
-
Rusting of iron (Fe + O2 → Fe2O3): This is a spontaneous reaction at room temperature because ΔG is negative. However, it's a relatively slow process.
-
Burning of methane (CH4 + O2 → CO2 + H2O): This is a highly spontaneous and rapid reaction due to a large negative ΔG and low activation energy.
-
Melting of ice (H2O(s) → H2O(l)): This is spontaneous above 0°C because the increase in entropy overcomes the endothermic nature of the reaction.
Conclusion
Determining if a reaction is spontaneous is a critical aspect of chemistry with broad implications. While the Gibbs Free Energy equation provides the fundamental framework, practical application involves considering standard free energy changes, equilibrium constants, electrochemical cell potentials, and even qualitative observations. Remember that spontaneity predicts possibility, not rate. Kinetic factors play a crucial role in determining the actual speed of a reaction. A thorough understanding of both thermodynamics and kinetics is essential for a complete comprehension of reaction behavior.
Latest Posts
Latest Posts
-
A Compound Contains Only Carbon Hydrogen And Oxygen
May 09, 2025
-
The Price Of An Item Was Lowered By 25
May 09, 2025
-
2 3 4 As A Improper Fraction
May 09, 2025
-
An Activity Series Of Metals Orders Metals By Their
May 09, 2025
-
Is Gravity Positive Or Negative In Free Fall
May 09, 2025
Related Post
Thank you for visiting our website which covers about How To Tell If A Reaction Is Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.