How To Find Concentration From Titration
listenit
Mar 24, 2025 · 6 min read
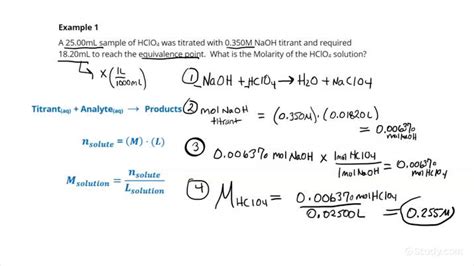
Table of Contents
How to Find Concentration from Titration: A Comprehensive Guide
Titration is a crucial analytical technique used in chemistry to determine the concentration of an unknown solution, called the analyte, by reacting it with a solution of known concentration, called the titrant. Understanding how to calculate concentration from titration data is fundamental to many chemical analyses. This comprehensive guide will walk you through the process, covering various titration types and providing practical examples.
Understanding the Fundamentals of Titration
Before diving into calculations, let's solidify our understanding of the underlying principles. Titration involves the gradual addition of the titrant to the analyte until the reaction is complete, a point known as the equivalence point. This point is usually indicated by a change in color using an indicator, signifying that the moles of titrant added are stoichiometrically equivalent to the moles of analyte present.
Key Concepts:
- Equivalence Point: The point in the titration where the moles of titrant added are exactly equal to the moles of analyte present. This is the theoretical endpoint.
- Endpoint: The point in the titration where the indicator changes color, signaling the approximate equivalence point. A slight discrepancy can exist between the equivalence point and endpoint.
- Molarity (M): The concentration of a solution expressed as moles of solute per liter of solution (mol/L).
- Stoichiometry: The quantitative relationship between reactants and products in a chemical reaction. This is crucial for determining the mole ratio between the titrant and analyte.
Types of Titration
Several types of titrations exist, each suited to specific analytes and reactions. The most common include:
1. Acid-Base Titration:
This is the most frequently encountered type, involving the reaction between an acid and a base. The equivalence point is reached when the moles of H⁺ ions from the acid equal the moles of OH⁻ ions from the base. Strong acid-strong base titrations are relatively straightforward, while weak acid-strong base or strong acid-weak base titrations require a slightly more nuanced approach.
2. Redox Titration:
These titrations involve oxidation-reduction reactions, where electrons are transferred between the titrant and analyte. Examples include permanganate titrations (using KMnO₄) and iodometric titrations (using I₂ or I⁻). The calculations involve balancing redox reactions and considering the number of electrons transferred.
3. Precipitation Titration:
In precipitation titrations, the reaction between the titrant and analyte forms an insoluble precipitate. An example is the titration of chloride ions (Cl⁻) with silver nitrate (AgNO₃) to form silver chloride (AgCl), a white precipitate. The calculations involve considering the stoichiometry of the precipitation reaction.
4. Complexometric Titration:
These titrations involve the formation of a complex ion between the titrant and analyte. A common example is the EDTA titration, used to determine the concentration of metal ions. The calculations are based on the stoichiometry of the complex formation reaction.
Calculating Concentration from Titration Data: A Step-by-Step Guide
The core principle behind calculating concentration from titration data involves using the stoichiometry of the reaction and the volume and concentration of the titrant used to reach the equivalence point.
General Steps:
-
Balanced Chemical Equation: Write a balanced chemical equation for the reaction between the titrant and analyte. This is crucial for determining the mole ratio.
-
Volume and Concentration of Titrant: Record the volume (in liters) of titrant used to reach the equivalence point and its known concentration (in molarity).
-
Moles of Titrant: Calculate the moles of titrant used using the formula: Moles = Molarity × Volume (in liters)
-
Mole Ratio: Use the balanced chemical equation to determine the mole ratio between the titrant and analyte. This ratio will be used to calculate the moles of analyte.
-
Moles of Analyte: Use the mole ratio to calculate the moles of analyte present in the sample.
-
Volume of Analyte: Record the volume (in liters) of the analyte solution used.
-
Molarity of Analyte: Finally, calculate the molarity of the analyte using the formula: Molarity = Moles of Analyte / Volume of Analyte (in liters)
Example: Acid-Base Titration
Let's consider a specific example of an acid-base titration.
Problem: 25.00 mL of an unknown concentration of NaOH solution is titrated with 0.100 M HCl solution. The equivalence point is reached when 20.00 mL of HCl is added. Calculate the concentration of the NaOH solution.
Solution:
-
Balanced Equation: NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l) The mole ratio between NaOH and HCl is 1:1.
-
Moles of HCl: Moles of HCl = 0.100 M × 0.0200 L = 0.00200 mol
-
Mole Ratio: From the balanced equation, the mole ratio of NaOH to HCl is 1:1. Therefore, moles of NaOH = moles of HCl = 0.00200 mol.
-
Molarity of NaOH: Molarity of NaOH = 0.00200 mol / 0.02500 L = 0.0800 M
Therefore, the concentration of the NaOH solution is 0.0800 M.
Addressing Potential Errors and Challenges
Several factors can introduce errors in titration, impacting the accuracy of the concentration determination. These include:
-
Indicator Error: The endpoint might not perfectly coincide with the equivalence point, leading to a slight deviation in the calculated concentration. Using the correct indicator for the specific titration is crucial.
-
Parallax Error: Incorrect reading of the burette due to parallax can significantly affect the volume measurement. Reading the burette at eye level is essential to minimize this error.
-
Improper Mixing: Insufficient mixing of the analyte and titrant can lead to an uneven reaction and inaccurate results. Gentle swirling or stirring is necessary throughout the titration.
-
Incomplete Reaction: If the reaction between the titrant and analyte is slow or incomplete, the calculated concentration will be inaccurate. Ensuring sufficient reaction time and appropriate conditions is important.
-
Impurities in Solutions: The presence of impurities in either the titrant or analyte can affect the results. Using high-purity chemicals is recommended.
Advanced Titration Techniques and Considerations
Beyond the basic principles, several advanced techniques and considerations enhance the accuracy and applicability of titration:
-
pH Titration Curves: Plotting the pH against the volume of titrant added creates a titration curve. This curve helps identify the equivalence point more precisely, especially in weak acid-weak base titrations.
-
Potentiometric Titration: This technique employs an electrode to measure the potential difference during the titration, providing a more accurate determination of the equivalence point than visual indicators.
-
Back Titration: Used when the reaction between the titrant and analyte is slow or incomplete. An excess of titrant is added, and then a second titration is performed to determine the amount of excess titrant.
Conclusion
Titration is a powerful analytical technique offering a precise method for determining the concentration of unknown solutions. By understanding the fundamental principles, mastering the calculation steps, and being aware of potential sources of error, you can confidently and accurately determine concentrations through titration, a cornerstone of quantitative chemical analysis. Remember, meticulous laboratory practices and careful attention to detail are crucial for obtaining reliable results.
Latest Posts
Latest Posts
-
Convert 4 1 2 To A Decimal
Mar 26, 2025
-
Where Is The Most Freshwater On Earth Located
Mar 26, 2025
-
Where Does Dna Replication Take Place In A Eukaryotic Cell
Mar 26, 2025
-
Salt Dissolves In Water Physical Or Chemical
Mar 26, 2025
-
Polygon With 4 Sides And 4 Angles
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Find Concentration From Titration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
