How To Find Activation Energy Of Reverse Reaction
listenit
Mar 16, 2025 · 6 min read
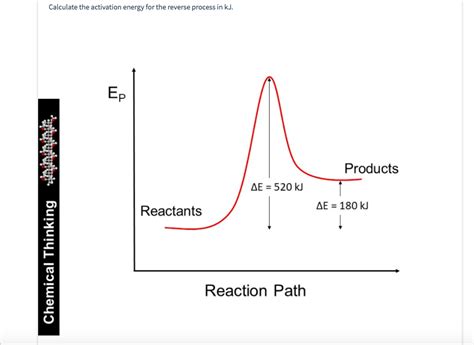
Table of Contents
How to Find the Activation Energy of a Reverse Reaction
Determining the activation energy (Ea) of a reverse reaction is crucial in understanding reaction kinetics and thermodynamics. While the activation energy of the forward reaction is often readily available or easier to measure experimentally, the activation energy of the reverse reaction isn't directly obtained in the same way. This article will delve into the various methods used to calculate the activation energy of a reverse reaction, explaining the underlying principles and offering practical examples.
Understanding Activation Energy and its Relationship to the Reverse Reaction
Before we dive into the methods, let's establish a firm understanding of activation energy. Activation energy is the minimum amount of energy required for a reaction to occur. It represents the energy barrier that reactants must overcome to transform into products. This barrier is related to the transition state, an unstable, high-energy intermediate state between reactants and products.
The reverse reaction, as the name suggests, is the reaction proceeding in the opposite direction—from products back to reactants. It also has its own activation energy, denoted as Ea(reverse). Importantly, Ea(forward) and Ea(reverse) are not equal, except in the very rare case of a perfectly symmetrical reaction energy profile. The difference between the two activation energies is directly related to the enthalpy change (ΔH) of the reaction.
Methods to Determine the Activation Energy of the Reverse Reaction
There are several ways to calculate the activation energy of the reverse reaction:
1. Using the Arrhenius Equation and the Equilibrium Constant
The Arrhenius equation describes the temperature dependence of the rate constant (k) of a reaction:
k = A * exp(-Ea/RT)
Where:
- k is the rate constant
- A is the pre-exponential factor (frequency factor)
- Ea is the activation energy
- R is the ideal gas constant
- T is the temperature in Kelvin
For a reversible reaction, we have two rate constants: k(forward) and k(reverse). The equilibrium constant (K) is the ratio of these rate constants:
K = k(forward) / k(reverse)
By knowing the equilibrium constant at different temperatures and using the van 't Hoff equation (which is derived from the Arrhenius equation and relates K to temperature), we can determine the enthalpy change (ΔH) of the reaction. Crucially, the relationship between the activation energies and enthalpy change is:
Ea(reverse) = Ea(forward) + ΔH
This equation provides a direct link between the forward and reverse activation energies. If we know the forward activation energy and the enthalpy change, we can easily calculate the activation energy of the reverse reaction.
Example:
Let's say the forward activation energy (Ea(forward)) is 50 kJ/mol, and the enthalpy change (ΔH) is -20 kJ/mol (exothermic reaction). Then:
Ea(reverse) = 50 kJ/mol + (-20 kJ/mol) = 30 kJ/mol
2. Experimental Determination of the Reverse Rate Constant
This method relies on directly measuring the rate constant of the reverse reaction at different temperatures. This can be challenging, particularly if the reverse reaction is slow or if the equilibrium lies far to one side.
However, if we can experimentally determine k(reverse) at several temperatures, we can then use the Arrhenius equation (applied to the reverse reaction) to calculate Ea(reverse). This involves plotting ln(k(reverse)) versus 1/T. The slope of the resulting line will be -Ea(reverse)/R.
Challenges: This method requires precise measurements of the reverse reaction rate, which can be difficult to achieve in practice, especially for fast reverse reactions or those that are close to equilibrium. Careful experimental design and advanced techniques are often necessary.
3. Computational Methods
Computational chemistry offers powerful tools to determine activation energies. Methods like Density Functional Theory (DFT) and other quantum mechanical calculations can model the reaction pathway, identifying the transition state and calculating the activation energies for both forward and reverse reactions. These methods require sophisticated software and expertise but can provide highly accurate results, even for complex reactions.
Advantages: Computational methods can be applied to systems where experimental measurements are difficult or impossible. They can also provide detailed information about the reaction mechanism and the structure of the transition state.
Limitations: The accuracy of computational methods depends heavily on the chosen computational level and the quality of the input parameters. The computational cost can also be significant for large systems.
4. Using Transition State Theory
Transition state theory provides a theoretical framework for understanding reaction rates and activation energies. It proposes that the reaction proceeds through a transition state, an unstable intermediate configuration. The activation energy is related to the free energy difference between the reactants and the transition state. While not directly providing a simple formula for calculating Ea(reverse) from Ea(forward), it offers a deeper understanding of the factors influencing activation energies.
By computationally modeling the transition state and using transition state theory, one can obtain the activation energies for both the forward and reverse reactions. This approach is particularly powerful when experimental data is scarce or difficult to obtain.
Practical Considerations and Applications
The choice of method for determining Ea(reverse) depends on several factors:
- Availability of data: If the forward activation energy and enthalpy change are known, the first method is the simplest and most efficient.
- Reaction kinetics: If the reverse reaction is readily observable and its rate can be measured accurately, experimental determination is feasible.
- Computational resources: If experimental data is limited or the system is complex, computational methods offer a viable alternative.
- Accuracy requirements: The accuracy requirements dictate the sophistication of the chosen method and the resources needed.
Knowledge of the reverse activation energy has significant implications in various fields:
- Catalysis: Understanding both forward and reverse activation energies is essential for designing efficient catalysts. A catalyst lowers the activation energy of both the forward and reverse reactions, accelerating the overall reaction rate and shifting the equilibrium.
- Chemical kinetics: The knowledge of both activation energies helps build more complete and accurate kinetic models, which are essential for understanding and predicting reaction behaviour.
- Chemical thermodynamics: The relationship between the activation energies and the enthalpy change provides valuable insights into the reaction thermodynamics.
- Industrial processes: In many industrial processes, controlling reaction rates and equilibrium is crucial for maximizing yield and efficiency. Knowing the activation energies of both forward and reverse reactions plays a key role in process optimization.
Conclusion
Determining the activation energy of a reverse reaction is a critical aspect of understanding reaction kinetics and thermodynamics. Multiple approaches exist, each with its own strengths and weaknesses. The choice of method depends on the specific circumstances, the availability of data, and the required accuracy. By employing these techniques appropriately, scientists and engineers can gain a more complete understanding of chemical reactions and improve the design of chemical processes. The integration of experimental methods with computational techniques often provides the most robust and comprehensive approach for accurately determining the activation energy of a reverse reaction.
Latest Posts
Latest Posts
-
What Is The Square Root Of 130
Mar 16, 2025
-
What Is The Lcm Of 2 And 8
Mar 16, 2025
-
How To Convert Rev Sec To Rad Sec
Mar 16, 2025
-
What Is 65 In Fraction Form
Mar 16, 2025
-
Which Of The Following Atoms Has The Largest Atomic Radius
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How To Find Activation Energy Of Reverse Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
