How Much Electrons Does Carbon Have
listenit
May 10, 2025 · 6 min read
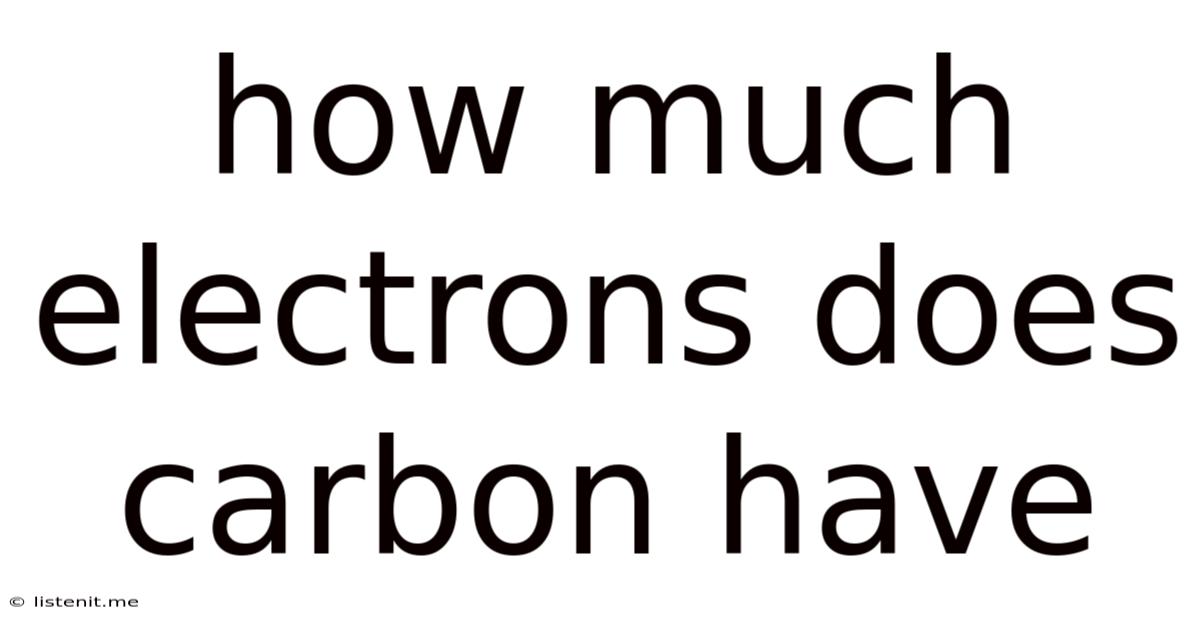
Table of Contents
How Many Electrons Does Carbon Have? A Deep Dive into Atomic Structure
Carbon, the backbone of life and a cornerstone of modern materials science, holds a fascinating place in the periodic table. Understanding its electronic structure is crucial to comprehending its unique properties and diverse applications. This comprehensive article delves into the intricacies of carbon's electron configuration, exploring its implications in chemistry, physics, and beyond. We'll move beyond simply stating the number of electrons to explore why it has that number and what that means for its behavior.
The Basics: Atomic Number and Electron Count
The simplest answer to the question, "How many electrons does carbon have?" is six. This is directly derived from its atomic number, which is also six. The atomic number represents the number of protons in an atom's nucleus, and in a neutral atom, the number of protons always equals the number of electrons. Therefore, a neutral carbon atom possesses six protons and six electrons.
Isotopes and Electron Count
While the number of protons defines an element, the number of neutrons can vary, leading to isotopes. Isotopes of carbon, such as carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C), all have six protons and therefore six electrons in their neutral states. The difference lies in the number of neutrons in their nuclei, affecting their mass but not their fundamental chemical behavior (mostly).
Electron Shell Configuration: The Key to Carbon's Reactivity
The significance of having six electrons extends far beyond a simple count. The arrangement of these electrons in energy levels, or electron shells, dictates carbon's remarkable chemical behavior. Carbon's electron configuration is 1s²2s²2p². Let's break this down:
- 1s²: The first shell (n=1) contains the lowest energy level and can hold a maximum of two electrons. These two electrons fill the 1s orbital.
- 2s²: The second shell (n=2) also includes an s orbital, which can accommodate another two electrons. These fill the 2s orbital.
- 2p²: The second shell also contains three p orbitals (2px, 2py, 2pz), each capable of holding two electrons. In carbon, only two of these p orbitals are occupied, each with one electron.
This specific arrangement is the foundation of carbon's extraordinary ability to form diverse and complex molecules. The presence of four valence electrons – those in the outermost shell (n=2) – allows carbon to form strong covalent bonds with other atoms.
Valence Electrons: The Driving Force Behind Bonding
Valence electrons are the electrons involved in chemical bonding. Carbon's four valence electrons (two in the 2s orbital and two in the 2p orbitals) are highly reactive and readily participate in the formation of covalent bonds. This ability to form four strong covalent bonds is responsible for carbon's unparalleled capacity to create a vast array of molecules, forming the basis of organic chemistry.
Carbon's Bonding Prowess: Single, Double, and Triple Bonds
The four valence electrons allow carbon to form single, double, and triple bonds with other atoms. This versatility leads to the formation of linear, branched, and ring structures, contributing to the immense diversity of organic compounds.
-
Single Bonds: Carbon forms a single covalent bond by sharing one electron with another atom. This is represented by a single line in structural formulas (e.g., C-C, C-H). Methane (CH₄) is a prime example, with carbon forming four single bonds with four hydrogen atoms.
-
Double Bonds: A double bond involves the sharing of two electron pairs between two atoms. This results in a stronger bond than a single bond, as seen in ethene (C₂H₄).
-
Triple Bonds: A triple bond is formed by the sharing of three electron pairs, resulting in an even stronger bond, as in ethyne (C₂H₂).
The ability to form these different types of bonds contributes significantly to the diverse structural possibilities and the vast range of properties exhibited by carbon-based molecules.
Carbon's Allotropes: Diverse Forms with Different Properties
Carbon's electron configuration also contributes to its existence in various allotropes – different structural forms of the same element. The most common allotropes are diamond, graphite, and fullerenes (including buckminsterfullerene, or "buckyballs").
-
Diamond: In diamond, each carbon atom is bonded to four other carbon atoms in a strong, three-dimensional tetrahedral network. This rigid structure accounts for diamond's hardness and high refractive index.
-
Graphite: In graphite, carbon atoms are arranged in layers of hexagonal networks. The bonds within each layer are strong, but the bonds between layers are weak, allowing them to slide past one another, giving graphite its lubricating properties.
-
Fullerenes: Fullerenes consist of carbon atoms arranged in spherical or tubular structures. Their unique shapes and properties have led to applications in nanotechnology and materials science.
The differences in the bonding and arrangement of carbon atoms in these allotropes directly impact their macroscopic properties. This exemplifies how a simple change in electron arrangement can dramatically affect an element's physical and chemical characteristics.
Carbon's Role in Organic Chemistry and Biochemistry
Carbon's unique ability to form four strong covalent bonds is the cornerstone of organic chemistry, the study of carbon-containing compounds. This ability allows carbon to form long chains, branched structures, and rings, creating the incredible diversity of molecules found in living organisms and synthetic materials.
In biochemistry, carbon is the central element of life. Carbohydrates, lipids, proteins, and nucleic acids – the four major classes of biomolecules – are all based on carbon skeletons. The specific arrangements of carbon atoms and their functional groups determine the properties and functions of these biomolecules, driving the complex processes of life.
Carbon's Importance in Materials Science
Beyond its biological significance, carbon also plays a crucial role in materials science. Its allotropes exhibit a wide range of properties, leading to applications in various fields:
- Diamond: Its hardness makes it ideal for cutting tools and abrasives.
- Graphite: Its lubricating properties and electrical conductivity are utilized in pencils, lubricants, and electrodes.
- Fullerenes: Their unique shapes and properties are explored for applications in drug delivery, electronics, and materials reinforcement.
- Carbon nanotubes: These cylindrical structures possess exceptional strength and electrical conductivity, finding applications in advanced composites and electronics.
- Graphene: A single layer of graphite, graphene exhibits extraordinary electrical conductivity, thermal conductivity, and strength. Its potential applications are vast and span electronics, energy storage, and composites.
These examples highlight the immense versatility of carbon and its importance in modern materials science.
Conclusion: The Significance of Six Electrons
The seemingly simple answer – carbon has six electrons – belies the profound implications of this fact. The specific arrangement of these six electrons in its electron shells dictates carbon's remarkable bonding capabilities, leading to the immense diversity of organic compounds and the wide range of applications in materials science and technology. From the intricate molecules of life to the cutting-edge materials of the future, carbon's six electrons are fundamentally responsible for its crucial role in our world. Understanding its electronic structure is key to unlocking its potential and further developing its applications in diverse fields.
Latest Posts
Latest Posts
-
What Does A Spontaneous Reaction Mean
May 10, 2025
-
Two Or More Atoms Held Together By A Chemical Bond
May 10, 2025
-
How Do You Determine The Shape Of A Molecule
May 10, 2025
-
How Many Valence Electrons In Xe
May 10, 2025
-
What Is The Density Of Ethyl Alcohol
May 10, 2025
Related Post
Thank you for visiting our website which covers about How Much Electrons Does Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.