How Many Valence Electrons In Zinc
listenit
Mar 20, 2025 · 6 min read
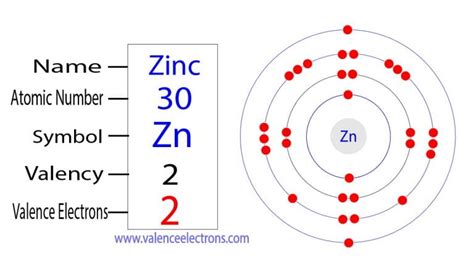
Table of Contents
How Many Valence Electrons Does Zinc Have? Understanding Zinc's Electronic Structure
Zinc, a ubiquitous element vital for numerous biological processes and industrial applications, presents a fascinating case study in electron configuration. Understanding its valence electrons – the outermost electrons involved in chemical bonding – is key to comprehending its reactivity and properties. This in-depth article explores the number of valence electrons in zinc, delves into its electronic structure, and explains how this impacts its chemical behavior.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in zinc, let's establish a foundational understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely bound to the nucleus and, consequently, are the ones most likely to participate in chemical reactions and bond formation with other atoms. The number of valence electrons significantly dictates an element's chemical properties, determining its reactivity, bonding capacity, and the types of compounds it can form.
Zinc's Position on the Periodic Table
The periodic table is a powerful tool for predicting an element's properties, including its valence electron count. Zinc (Zn), with an atomic number of 30, resides in period 4 and group 12 (formerly IIB). Its placement within the d-block of the periodic table suggests a complex electronic structure, but the group 12 position provides a clue to its valence electron configuration. Elements within this group generally exhibit a +2 oxidation state, implying two valence electrons.
Zinc's Electronic Configuration: Unveiling the Mystery
To definitively determine the number of valence electrons in zinc, we need to examine its electronic configuration. This configuration describes how electrons are distributed among the various energy levels and sublevels within the atom. Zinc's electronic configuration is written as 1s²2s²2p⁶3s²3p⁶3d¹⁰4s².
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the filled inner shells, also known as core electrons. These electrons are tightly bound to the nucleus and generally do not participate in chemical bonding.
-
3d¹⁰: This represents the filled 3d subshell. While these electrons are relatively close in energy to the outermost electrons, they are typically considered core electrons in zinc's case due to their stable filled configuration.
-
4s²: This represents the outermost shell, containing two electrons. These are the valence electrons.
Therefore, despite zinc's presence in the d-block, the 4s electrons are the only ones actively participating in chemical bonding, making zinc have two valence electrons.
Why are the 3d electrons not considered valence electrons in Zinc?
While the 3d subshell is energetically close to the 4s subshell, the filled 3d¹⁰ configuration is exceptionally stable. This stability implies a reluctance of the 3d electrons to participate in chemical reactions. The filled d-orbital provides a shielding effect, further reducing the 3d electrons' involvement in bonding. Therefore, the chemical behavior of zinc is primarily governed by the two electrons in the 4s subshell.
Implications of Zinc's Two Valence Electrons: Chemical Behavior and Properties
The fact that zinc possesses only two valence electrons significantly influences its chemical behavior and physical properties. This explains:
1. Oxidation State:
Zinc almost invariably exhibits a +2 oxidation state. This means it readily loses its two valence electrons to achieve a stable, completely filled 3d¹⁰ electron configuration. This tendency to lose two electrons is the driving force behind most of zinc's chemical reactions.
2. Reactivity:
Compared to alkali metals and alkaline earth metals with one and two valence electrons respectively, zinc is relatively unreactive. The relatively higher ionization energy needed to remove electrons and the stability of the resulting Zn²⁺ ion contribute to this. While it's not inert, its reactions are often slower than those of more reactive metals.
3. Bonding:
Zinc's bonding behavior is largely dictated by its two valence electrons. It predominantly forms ionic bonds, donating its two valence electrons to electronegative elements such as oxygen, chlorine, and sulfur. It can also participate in coordinate covalent bonding, acting as a Lewis acid (electron acceptor).
4. Metallic Properties:
Zinc is a transition metal, exhibiting typical metallic properties such as good electrical conductivity and malleability. These properties are related to the delocalized electrons in the metallic lattice, including valence electrons which contribute to the electron sea model.
Zinc in Biological Systems: The Importance of Valence Electrons
Zinc's biological significance stems directly from its chemical properties, primarily governed by its two valence electrons. It plays vital roles in various enzymatic processes, acting as a cofactor or structural component in numerous enzymes. The specific way zinc interacts with these enzymes is directly linked to its ability to donate or accept electron pairs, a characteristic stemming from its valence electron configuration.
Some key roles of zinc in biological systems include:
- Enzyme activity: Zinc is a crucial component in many enzymes involved in crucial metabolic pathways, DNA replication, and RNA synthesis. Its ability to coordinate with other molecules through its valence electrons is critical for enzyme function.
- Gene expression: Zinc finger proteins, a crucial class of transcription factors, rely on zinc ions bound within specific structural motifs to regulate gene expression. These interactions are mediated by zinc's coordination chemistry, governed by the availability of its two valence electrons.
- Immune function: Zinc plays a significant role in immune function, impacting cell growth, proliferation, and differentiation of immune cells.
- Wound healing: Zinc is essential for proper wound healing, supporting collagen synthesis and inflammatory responses.
Zinc in Industrial Applications: From Galvanization to Alloys
Zinc's industrial applications leverage its unique properties, rooted in its electronic structure and the presence of two valence electrons. Some prominent examples include:
- Galvanization: Zinc's protective coating over steel prevents corrosion by sacrificial protection. Zinc readily oxidizes, protecting the underlying steel from oxidation. This behavior is a direct consequence of its tendency to lose two valence electrons.
- Alloys: Zinc is a constituent in various alloys, improving their mechanical properties, corrosion resistance, and castability. Brass (copper-zinc alloy) and zinc-aluminum alloys illustrate the importance of zinc in metallurgy.
- Batteries: Zinc is employed in batteries as the anode (negative electrode) material, facilitating electrical energy production by oxidation of zinc. The release of two valence electrons is the basis of this electrochemical process.
Conclusion: The Significance of Zinc's Two Valence Electrons
In conclusion, zinc possesses two valence electrons located in its 4s subshell. This seemingly simple fact has profound implications across diverse fields. From its reactivity and bonding behavior to its crucial roles in biological systems and numerous industrial applications, the chemical properties of zinc are directly shaped by the presence and behavior of these two outermost electrons. Understanding zinc's electronic structure, particularly its valence electron configuration, is fundamental to appreciating its significance in both the natural world and technological advancements. The stable, filled d-orbital further contributes to its unique behavior, ensuring its importance continues to be explored and utilized in various applications.
Latest Posts
Latest Posts
-
What Is The Difference Between A Federalist And An Anti Federalist
Mar 20, 2025
-
What Is The Si Unit Of Power
Mar 20, 2025
-
What Percent Of 18 Is 24
Mar 20, 2025
-
What Is 4 Percent Of 50
Mar 20, 2025
-
How To Find The Ph At Equivalence Point
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Zinc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
