How Many Valence Electrons In Silicon
listenit
Mar 14, 2025 · 5 min read
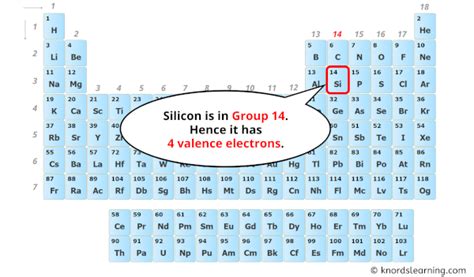
Table of Contents
How Many Valence Electrons in Silicon? Understanding Silicon's Bonding Behavior
Silicon, the second most abundant element in the Earth's crust, plays a crucial role in modern technology, forming the backbone of countless electronic devices. Understanding its properties, particularly its valence electrons, is key to comprehending its behavior and applications. This comprehensive guide delves into the world of silicon, explaining its electronic structure and how its four valence electrons contribute to its unique bonding capabilities and semiconductor properties.
What are Valence Electrons?
Before diving into silicon's specifics, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (valence shell) of an atom. These electrons are the most loosely held and, therefore, are the primary participants in chemical bonding. They determine an atom's reactivity and the types of bonds it can form—covalent, ionic, or metallic. The number of valence electrons significantly influences an element's chemical and physical properties.
Silicon's Electronic Configuration: Unveiling the Four Valence Electrons
Silicon's atomic number is 14, indicating it possesses 14 protons and 14 electrons in a neutral atom. To understand its valence electrons, we need to examine its electronic configuration. This configuration describes how electrons are distributed among different energy levels or shells within the atom.
Silicon's electronic configuration is 1s²2s²2p⁶3s²3p².
-
1s², 2s², 2p⁶: These inner shell electrons are tightly bound to the nucleus and are not involved in chemical bonding. They are considered core electrons.
-
3s²3p²: These electrons reside in the outermost shell (the third energy level), making them the valence electrons. There are two electrons in the 3s subshell and two electrons in the 3p subshell, totaling four valence electrons.
Therefore, silicon has four valence electrons. This crucial detail explains its behavior in forming covalent bonds and its semiconducting properties.
The Significance of Four Valence Electrons: Covalent Bonding in Silicon
Silicon's four valence electrons are instrumental in its ability to form strong covalent bonds. A covalent bond involves the sharing of electrons between atoms to achieve a stable electron configuration, typically resembling that of a noble gas (a full outer shell).
Silicon atoms readily share their four valence electrons with four other silicon atoms, creating a tetrahedral structure. Each silicon atom is bonded to four neighboring silicon atoms, forming a three-dimensional network. This extensive network is responsible for silicon's strength and crystalline structure.
Silicon's Covalent Bonding in Detail:
-
Sharing Electrons: Each silicon atom shares one electron with each of its four neighbors, resulting in a shared pair of electrons between each pair of silicon atoms. This sharing completes the octet rule (eight electrons in the outermost shell) for each silicon atom, making the structure very stable.
-
Tetrahedral Geometry: The four covalent bonds arrange themselves in a three-dimensional tetrahedral geometry around each silicon atom. This arrangement maximizes the distance between the bonding electrons, minimizing repulsion and leading to a stable configuration.
-
Strong Bonds: These covalent bonds are relatively strong, leading to silicon's high melting point (1414 °C) and hardness. The strength of these bonds directly relates to the strength of the overall silicon crystal structure.
Silicon's Semiconducting Properties: A Consequence of Valence Electrons
Silicon's four valence electrons are not only crucial for its bonding but also for its remarkable semiconducting properties. Semiconductors are materials with electrical conductivity between that of conductors (like copper) and insulators (like rubber).
Understanding Silicon's Semiconductivity:
-
Energy Gap: In silicon, the four valence electrons participate in strong covalent bonds, creating a filled valence band. However, there is an energy gap between the valence band and the conduction band (the energy level where electrons can move freely and conduct electricity). This energy gap is relatively small compared to insulators.
-
Excitation of Electrons: At absolute zero temperature, silicon behaves as an insulator. However, at room temperature, some electrons gain enough thermal energy to overcome the energy gap and jump to the conduction band. These excited electrons can then contribute to electrical conductivity.
-
Doping and Conductivity Control: The electrical conductivity of silicon can be precisely controlled by doping – adding small amounts of impurity atoms with either three or five valence electrons. This doping process alters the number of charge carriers (electrons or holes) in the silicon, significantly influencing its conductivity, making it suitable for creating transistors and integrated circuits.
Silicon's Applications: A Testament to Its Unique Properties
Silicon's unique properties, stemming directly from its four valence electrons, have revolutionized technology. Its applications are vast and continue to expand.
-
Semiconductors: Silicon is the cornerstone of the semiconductor industry, forming the basis for transistors, integrated circuits, microprocessors, and memory chips found in virtually every electronic device.
-
Solar Cells: Silicon's ability to absorb sunlight and generate electricity makes it an essential component of solar cells, contributing significantly to renewable energy technologies.
-
Ceramics and Glass: Silicon dioxide (SiO2), commonly known as silica, is a key ingredient in many ceramics and glasses, contributing to their strength, durability, and thermal properties.
-
Silicone Polymers: Silicon-based polymers, known as silicones, possess unique properties like flexibility, water resistance, and thermal stability, making them suitable for a wide range of applications including sealants, lubricants, and medical implants.
Conclusion: The Importance of Silicon's Four Valence Electrons
The presence of four valence electrons in silicon is not simply a fact of its atomic structure; it’s the fundamental reason behind its remarkable versatility and importance in modern technology. These four electrons drive its covalent bonding, leading to strong, stable crystalline structures. They also dictate its semiconducting behavior, making silicon the foundation of the electronics revolution. Understanding the implications of these four valence electrons is paramount for grasping the significance of silicon in our technologically advanced world. From the smallest microchip to the largest solar panel, silicon's properties, derived directly from its electronic configuration, shape our daily lives in countless ways. The exploration of silicon and its applications remains a dynamic and rapidly evolving field, with continuous innovation promising even more groundbreaking advancements in the future.
Latest Posts
Latest Posts
-
Least Common Multiple 15 And 9
Mar 14, 2025
-
8 Of What Number Is 2
Mar 14, 2025
-
How Many Valence Electrons Does An Atom Of Chlorine Have
Mar 14, 2025
-
At The Instant The Traffic Light Turns Green
Mar 14, 2025
-
Density Of Water In Lbm In 3
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
