How Many Valence Electrons Does Cl
listenit
Mar 28, 2025 · 5 min read
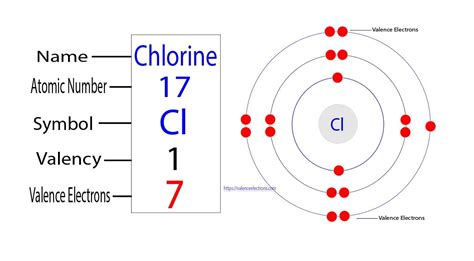
Table of Contents
How Many Valence Electrons Does Cl Have? A Deep Dive into Chlorine's Electronic Structure
Chlorine (Cl), a vital element in various aspects of our lives, from table salt to industrial processes, possesses a fascinating electronic structure. Understanding its valence electrons is crucial to comprehending its chemical behavior and reactivity. This article delves deep into the question: How many valence electrons does Cl have? We'll explore chlorine's electron configuration, its position on the periodic table, and how its valence electrons dictate its chemical bonding and properties.
Understanding Valence Electrons
Before we pinpoint the number of valence electrons in chlorine, let's establish a clear understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons directly influences an element's chemical properties, such as its electronegativity, ionization energy, and its tendency to gain or lose electrons to achieve a stable electron configuration.
Chlorine's Position on the Periodic Table
The periodic table provides a wealth of information about an element's properties, including its number of valence electrons. Chlorine (Cl) is located in Group 17 (also known as Group VIIA or the halogens) and Period 3. The group number (excluding transition metals) generally indicates the number of valence electrons. Therefore, elements in Group 17, like chlorine, typically have seven valence electrons.
Chlorine's Electron Configuration
The electron configuration provides a detailed picture of how electrons are distributed among the various energy levels and subshells within an atom. Chlorine's atomic number is 17, meaning it has 17 protons and 17 electrons in a neutral atom. Its electron configuration is 1s²2s²2p⁶3s²3p⁵.
Let's break this down:
- 1s²: Two electrons in the first energy level, first subshell (s subshell).
- 2s²: Two electrons in the second energy level, first subshell (s subshell).
- 2p⁶: Six electrons in the second energy level, second subshell (p subshell).
- 3s²: Two electrons in the third energy level, first subshell (s subshell).
- 3p⁵: Five electrons in the third energy level, second subshell (p subshell).
The outermost shell is the third energy level (n=3), containing the 3s and 3p electrons. Adding the electrons in the 3s and 3p subshells (2 + 5 = 7), we confirm that chlorine has seven valence electrons.
Why Seven Valence Electrons? The Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight electrons in their outermost shell, similar to the electron configuration of noble gases. Chlorine, with its seven valence electrons, is one electron short of achieving a stable octet. This explains its high reactivity and its strong tendency to gain an electron, forming a stable chloride ion (Cl⁻) with a full octet.
How Chlorine Achieves a Stable Octet
Chlorine readily achieves a stable octet by gaining one electron. This process is called reduction, and it results in the formation of a negatively charged chloride ion (Cl⁻). This negatively charged ion is significantly more stable than the neutral chlorine atom. The gain of an electron fills the 3p subshell, resulting in a full octet (3s²3p⁶) in the valence shell.
Chemical Bonding and Valence Electrons
The seven valence electrons of chlorine are pivotal in determining the types of chemical bonds it can form. Chlorine's strong tendency to gain an electron leads it to predominantly form ionic bonds with electropositive elements (metals). Ionic bonds involve the transfer of electrons from one atom to another, resulting in the formation of ions with opposite charges that are electrostatically attracted to each other. A classic example is the formation of sodium chloride (NaCl), or table salt, where chlorine gains an electron from sodium.
Chlorine can also form covalent bonds, sharing electrons with other nonmetals. In covalent bonds, atoms share electrons to achieve a stable octet. This is particularly common when chlorine bonds with other nonmetals that have a similar electronegativity. For instance, chlorine forms covalent bonds in molecules like chlorine gas (Cl₂), where two chlorine atoms share a pair of electrons to achieve a stable octet.
The Importance of Understanding Valence Electrons in Chlorine
Understanding the number of valence electrons in chlorine is essential for:
- Predicting chemical reactivity: The seven valence electrons explain chlorine's high reactivity and its tendency to form stable compounds.
- Understanding chemical bonding: The number of valence electrons dictates whether chlorine will form ionic or covalent bonds.
- Explaining chemical properties: Chlorine's electronegativity, ionization energy, and other properties are directly related to its valence electron configuration.
- Applications in chemistry and related fields: Knowledge of chlorine's valence electrons is crucial for understanding its applications in various fields, such as industrial chemistry, pharmaceuticals, and materials science.
Chlorine's Role in Everyday Life and Industry
Chlorine's reactivity and properties, dictated by its seven valence electrons, make it a vital element in many aspects of our daily lives and industrial processes. Here are some key examples:
- Water Treatment: Chlorine is widely used as a disinfectant in water treatment plants to kill harmful bacteria and viruses, ensuring safe drinking water.
- Bleach: Many household bleaches contain chlorine compounds, which are effective oxidizing agents that can remove stains and disinfect surfaces.
- Plastics and Polymers: Chlorine-containing compounds are used in the production of various plastics and polymers, which have numerous applications in packaging, construction, and other industries.
- Pharmaceuticals: Chlorine is present in many pharmaceuticals, playing crucial roles in their structure and function.
- Solvents and Refrigerants: Certain chlorine-containing compounds have been used as solvents and refrigerants, although their use is now restricted due to environmental concerns (e.g., ozone depletion).
Conclusion: The Significance of Seven Valence Electrons
In summary, chlorine (Cl) possesses seven valence electrons. This fundamental aspect of its electronic structure determines its chemical behavior, reactivity, and the types of bonds it can form. Understanding this crucial characteristic is essential for comprehending chlorine's role in various chemical processes, its widespread applications in different fields, and its overall significance in the world around us. From water purification to the synthesis of essential pharmaceuticals, chlorine’s seven valence electrons underpin its versatile and indispensable role in modern society. Further exploration into chlorine chemistry will continue to unveil its unique contributions to scientific advancements and technological innovations.
Latest Posts
Latest Posts
-
4 5 To The Power Of 2
Mar 31, 2025
-
Can You Determine The Activation Energy Of The Reverse Reaction
Mar 31, 2025
-
What Is 3 4 Of A Mile
Mar 31, 2025
-
Find The Limit Of A Sequence
Mar 31, 2025
-
How Many Atoms In One Mole
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
