How Many Valence Electrons Are In Silicon
listenit
Mar 16, 2025 · 5 min read
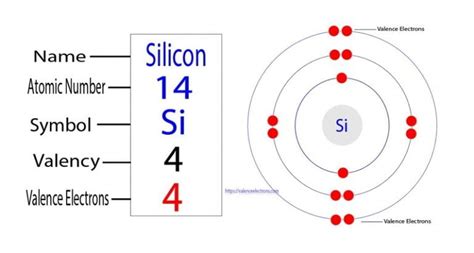
Table of Contents
How Many Valence Electrons Are in Silicon? A Deep Dive into Silicon's Electronic Structure
Silicon, the second most abundant element in the Earth's crust after oxygen, plays a crucial role in modern technology. Its unique electronic properties, particularly its valence electron configuration, are the foundation for its widespread use in semiconductors, solar cells, and countless other applications. Understanding the number of valence electrons in silicon is key to grasping its behavior and applications. This article will explore this fundamental aspect of silicon's atomic structure in detail, examining its implications for its chemical bonding and electrical conductivity.
What are Valence Electrons?
Before delving into silicon specifically, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the most loosely bound to the nucleus and are primarily responsible for the atom's chemical behavior and its ability to form chemical bonds with other atoms. They determine an element's reactivity and the type of bonds it can form – ionic, covalent, or metallic. The number of valence electrons directly influences an element's position in the periodic table and its group properties.
Determining Silicon's Valence Electrons
Silicon (Si) has an atomic number of 14, meaning a neutral silicon atom contains 14 protons and 14 electrons. To determine the number of valence electrons, we need to examine its electron configuration. Using the Aufbau principle and Hund's rule, we can fill the electron orbitals:
- 1s² 2s² 2p⁶ 3s² 3p²
This electron configuration tells us that:
- Shell 1 (n=1): Contains 2 electrons (1s²)
- Shell 2 (n=2): Contains 8 electrons (2s² 2p⁶)
- Shell 3 (n=3): Contains 4 electrons (3s² 3p²)
The outermost shell is the third shell (n=3), which contains 4 electrons. Therefore, silicon has 4 valence electrons.
The Significance of Silicon's Four Valence Electrons
The presence of four valence electrons is critical to silicon's properties and its technological importance. This number allows silicon to form stable covalent bonds with four other atoms, typically other silicon atoms or atoms of elements like oxygen, hydrogen, or carbon.
Covalent Bonding in Silicon
Silicon's four valence electrons enable it to participate in covalent bonding. In a covalent bond, atoms share electrons to achieve a stable electron configuration, usually a filled outer shell (octet rule). In silicon's case, each silicon atom shares its four valence electrons with four neighboring silicon atoms, forming a strong, three-dimensional network structure. This network is responsible for silicon's high melting point and hardness.
Semiconducting Properties
The tetrahedral bonding arrangement in silicon, a direct result of its four valence electrons, is fundamental to its semiconducting properties. At absolute zero temperature, silicon behaves as an insulator, with all its valence electrons tightly bound in covalent bonds. However, at higher temperatures, some electrons gain enough thermal energy to break free from their bonds, becoming mobile charge carriers (electrons) and leaving behind "holes" – positively charged vacancies. This allows silicon to conduct electricity, although not as efficiently as a conductor like copper.
Doping Silicon for Enhanced Conductivity
The semiconducting behavior of silicon can be precisely controlled by a process called doping. Doping involves introducing small amounts of impurity atoms with either three or five valence electrons into the silicon crystal lattice.
-
N-type doping: Introducing atoms with five valence electrons (like phosphorus or arsenic) leads to extra electrons in the silicon lattice, significantly increasing its conductivity. The extra electrons become the majority carriers.
-
P-type doping: Introducing atoms with three valence electrons (like boron or aluminum) creates "holes" in the silicon lattice, also increasing its conductivity. The holes become the majority carriers.
This ability to control silicon's conductivity through doping is crucial for creating transistors, integrated circuits, and other essential components of modern electronics.
Silicon's Role in Technology
Silicon's unique combination of properties, largely stemming from its four valence electrons, makes it indispensable in countless technological applications:
-
Semiconductors: Silicon is the cornerstone of the semiconductor industry, forming the basis of integrated circuits (ICs), microprocessors, and memory chips found in computers, smartphones, and countless other electronic devices.
-
Solar Cells: Silicon's ability to absorb sunlight and convert it into electricity makes it a vital component of photovoltaic cells (solar cells). The generation of electron-hole pairs upon light absorption facilitates the flow of electric current.
-
Sensors: Silicon-based sensors are used in a wide range of applications, including temperature sensors, pressure sensors, and accelerometers. These sensors often rely on silicon's ability to change its electrical properties in response to environmental stimuli.
-
Coatings and Materials: Silicon-containing compounds like silicones and silicates find applications as coatings, sealants, and structural materials due to their unique thermal and chemical stability.
Conclusion: The Importance of Understanding Valence Electrons
The simple number "four" – the number of valence electrons in silicon – holds immense significance in the realm of science and technology. It dictates silicon's chemical bonding behavior, its semiconducting nature, and its ability to be manipulated for diverse technological applications. A thorough understanding of silicon's electronic structure, specifically the role of its valence electrons, is crucial for continued innovation and advancements in various technological fields. From the microprocessors powering our computers to the solar cells harnessing the sun's energy, silicon’s four valence electrons underpin a technological revolution. The future of electronics and many other technological advancements are inextricably linked to our understanding and continued exploration of this fundamental aspect of silicon's atomic structure. Further research into materials science and nanotechnology promises even more innovative applications built upon the foundation laid by silicon’s four valence electrons. The exploration of silicon's behavior and manipulation continues to drive progress across a multitude of scientific and technological disciplines.
Latest Posts
Latest Posts
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
-
How Can Sedimentary Rock Become Metamorphic Rock
Mar 17, 2025
-
What Is The Square Root Of 500
Mar 17, 2025
-
What Is The Next Number In The Sequence 3 9 27 81
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Silicon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
