How Many Protons Does Titanium Have
listenit
Mar 21, 2025 · 5 min read
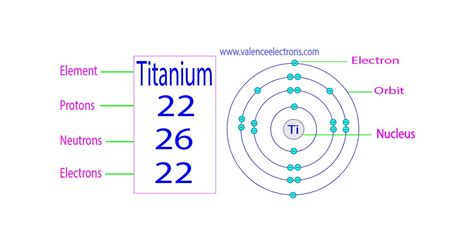
Table of Contents
- How Many Protons Does Titanium Have
- Table of Contents
- How Many Protons Does Titanium Have? A Deep Dive into Atomic Structure
- Understanding Atomic Structure: The Foundation of Elements
- The Answer: Titanium's Proton Count
- Isotopes: Variations on a Theme
- The Significance of Titanium's 22 Protons: Properties and Applications
- Applications Leveraging Titanium's Unique Properties
- Conclusion: The Defining Role of 22 Protons
- Latest Posts
- Latest Posts
- Related Post
How Many Protons Does Titanium Have? A Deep Dive into Atomic Structure
Titanium. The name conjures images of strong, lightweight alloys used in everything from aerospace engineering to medical implants. But what makes titanium, well, titanium? The answer lies deep within its atomic structure, specifically in the number of protons it possesses. This article will not only answer the central question – how many protons does titanium have? – but will delve into the broader concepts of atomic number, isotopes, and the implications of titanium's proton count for its unique properties.
Understanding Atomic Structure: The Foundation of Elements
Before we uncover the number of protons in titanium, let's establish a foundational understanding of atomic structure. Atoms, the fundamental building blocks of matter, consist of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutrally charged particles residing in the nucleus alongside protons. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom.
The atomic number of an element is crucial. It represents the number of protons in the nucleus of an atom of that element. This number uniquely identifies each element on the periodic table. It's the fundamental characteristic that distinguishes hydrogen from helium, oxygen from titanium, and so on. This number is unchanging; you can't have titanium with a different number of protons and still call it titanium.
The Answer: Titanium's Proton Count
So, how many protons does titanium have? Titanium (Ti) has 22 protons. This is its defining characteristic. Every atom that possesses 22 protons is, by definition, a titanium atom. This fundamental fact is reflected in its atomic number, which is 22. You'll find this number listed prominently on any periodic table alongside its symbol (Ti) and atomic weight.
Isotopes: Variations on a Theme
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with differing numbers of neutrons are called isotopes. Titanium has several naturally occurring isotopes, each with a different number of neutrons but always with 22 protons. These isotopes are often expressed as `${}^{A}\text{Ti}$, where A is the mass number (protons + neutrons).
For example:
- Titanium-46 (⁴⁶Ti): This isotope has 22 protons and 24 neutrons.
- Titanium-47 (⁴⁷Ti): This isotope has 22 protons and 25 neutrons.
- Titanium-48 (⁴⁸Ti): This isotope has 22 protons and 26 neutrons.
- Titanium-49 (⁴⁹Ti): This isotope has 22 protons and 27 neutrons.
- Titanium-50 (⁵⁰Ti): This isotope has 22 protons and 28 neutrons.
The abundance of each isotope varies in nature. Titanium-48 is the most abundant isotope, making up around 73.8% of naturally occurring titanium. The differences in neutron numbers subtly affect the mass and some nuclear properties, but the chemical properties remain largely consistent because the number of protons (and thus, electrons) remains constant.
The Significance of Titanium's 22 Protons: Properties and Applications
The 22 protons in titanium's nucleus are directly responsible for its unique properties, which lead to its widespread use in various industries. These properties include:
-
High Strength-to-Weight Ratio: Titanium is exceptionally strong yet remarkably lightweight, making it ideal for applications where minimizing weight is crucial, such as aerospace components and sporting equipment. This strength is a direct consequence of the electron configuration arising from its 22 protons and the resulting strong metallic bonding.
-
Corrosion Resistance: Titanium exhibits exceptional resistance to corrosion, even in harsh environments. This is due to the formation of a stable and protective oxide layer on its surface, a property inherently linked to its electron configuration determined by its 22 protons. This makes it suitable for marine applications, chemical processing equipment, and biomedical implants.
-
Biocompatibility: Titanium's excellent biocompatibility makes it a popular choice for medical implants. Its resistance to corrosion and its ability to integrate with bone tissue are vital for its success in this area. The inertness, stemming from its electron configuration influenced by its 22 protons, is critical to its biocompatibility.
-
High Melting Point: Titanium boasts a relatively high melting point, contributing to its strength and durability at elevated temperatures. This characteristic also results from the strong metallic bonding influenced by the arrangement of its electrons based on its 22 protons.
Applications Leveraging Titanium's Unique Properties
The combination of these properties fuels titanium's use in a wide array of applications:
-
Aerospace: Titanium alloys are extensively used in aircraft engines, airframes, and spacecraft due to their high strength-to-weight ratio and corrosion resistance.
-
Medical Implants: Titanium's biocompatibility allows for its use in joint replacements, dental implants, and other medical devices.
-
Chemical Processing: Titanium's corrosion resistance makes it suitable for equipment used in handling corrosive chemicals.
-
Sporting Goods: Titanium's strength and lightweight nature are utilized in high-performance sporting goods, such as bicycles, golf clubs, and tennis rackets.
-
Automotive: Increasingly, titanium is finding its way into high-performance automotive parts where weight reduction and enhanced durability are paramount.
-
Marine Engineering: The combination of strength and corrosion resistance makes titanium a valuable material in marine applications such as ship hulls, propellers, and underwater equipment.
Conclusion: The Defining Role of 22 Protons
In conclusion, titanium possesses 22 protons, a fact that fundamentally defines its identity and dictates its remarkable properties. This proton count determines its atomic number, influences its electron configuration, and ultimately dictates its strength, corrosion resistance, biocompatibility, and high melting point. These properties, in turn, are what make titanium such a valuable and versatile material across a multitude of industries. Understanding the significance of the 22 protons in titanium is crucial for appreciating its unique place in the world of materials science and engineering. Further research into its isotopes and alloying behavior expands our understanding of this extraordinary element and its potential for future applications. The seemingly simple answer—22 protons—unlocks a wealth of scientific and technological possibilities.
Latest Posts
Latest Posts
-
Net Primary Productivity Vs Gross Primary Productivity
Mar 28, 2025
-
How Many Ounces Is 2 L Of Water
Mar 28, 2025
-
1 2 3 4 How Many
Mar 28, 2025
-
Is Square Root Of 5 Irrational
Mar 28, 2025
-
What Do You Call A Pack Of Penguins
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Titanium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
