How Many Protons Are In Na
listenit
Mar 13, 2025 · 4 min read
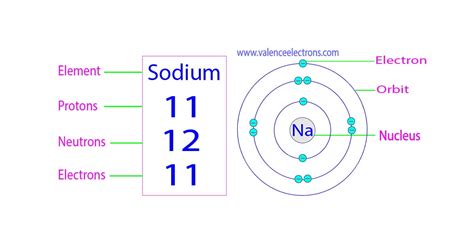
Table of Contents
How Many Protons Are in Na? Understanding Atomic Structure and Sodium
Determining the number of protons in an atom of sodium (Na) is fundamental to understanding its chemical properties and behavior. This seemingly simple question delves into the core principles of atomic structure and the periodic table, concepts vital in chemistry and related fields. This comprehensive guide will not only answer the question directly but also explore the broader context of atomic structure, isotopes, and the implications of proton number.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before diving into the specifics of sodium, let's establish a firm grasp of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutrally charged particles also located in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The Atomic Number and Sodium's Identity
The atomic number of an element is arguably its most crucial characteristic. It represents the number of protons found in the nucleus of a single atom of that element. This number uniquely identifies each element on the periodic table. Elements are arranged in order of increasing atomic number, reflecting the systematic increase in proton count.
For sodium (Na), the atomic number is 11. This definitive statement answers our primary question: there are 11 protons in a sodium atom.
Isotopes: Variations in Neutron Count
While the number of protons determines the element, the number of neutrons can vary. Atoms of the same element with differing neutron counts are called isotopes. Isotopes of a given element exhibit similar chemical properties due to the identical number of protons and electrons, but they may differ slightly in physical properties, such as mass and radioactive behavior.
Sodium has several known isotopes, each with a varying number of neutrons. The most common isotope, ²³Na, has 12 neutrons (23 total nucleons - 11 protons = 12 neutrons). Other isotopes exist but are less abundant.
Implications of Isotopes
The existence of isotopes affects various aspects of science and technology, including:
- Nuclear medicine: Certain isotopes are radioactive and used in medical imaging and treatments.
- Radioactive dating: Isotopic ratios are used to determine the age of geological samples and artifacts.
- Nuclear energy: Nuclear fission relies on specific isotopes like Uranium-235.
The Periodic Table: A Visual Representation of Atomic Structure
The periodic table is a powerful tool that organizes elements based on their atomic number and recurring chemical properties. The table's arrangement visually reflects the relationship between the number of protons and an element's position.
Sodium (Na), located in Group 1 (alkali metals), readily reveals its atomic number (11) by its position on the table. This arrangement highlights sodium's properties – its reactivity and tendency to lose one electron to achieve a stable electron configuration.
Electron Configuration and Chemical Bonding
The 11 protons in a sodium atom attract 11 electrons, which occupy specific energy levels or shells. The electron configuration of sodium is 1s²2s²2p⁶3s¹. This arrangement explains sodium's chemical reactivity. The single electron in the outermost shell (3s¹) is easily lost, forming a stable +1 ion (Na⁺). This tendency to lose an electron is crucial in understanding sodium's participation in chemical reactions and compound formation.
Ionic Bonding in Sodium Chloride
The most well-known example of sodium's chemical behavior is its reaction with chlorine (Cl) to form sodium chloride (NaCl), common table salt. Sodium readily donates its 3s¹ electron to chlorine, forming Na⁺ and Cl⁻ ions. These oppositely charged ions attract each other through ionic bonding, forming a stable crystalline structure.
Sodium's Importance in Biology and Industry
Sodium's unique properties, stemming directly from its 11 protons, make it essential in numerous biological and industrial applications:
-
Biological Roles: Sodium plays a vital role in nerve impulse transmission, muscle contraction, and fluid balance in living organisms. Its presence in bodily fluids and its participation in electrochemical gradients are fundamental to life processes.
-
Industrial Uses: Sodium is used in the production of various chemicals, including sodium hydroxide (NaOH) used in soap and paper manufacturing. It's also a component in many alloys and is used in the production of certain fuels.
Conclusion: The Significance of 11 Protons
The seemingly simple answer – 11 protons in a sodium atom – opens a vast landscape of understanding within chemistry and related fields. This number defines sodium's identity, dictates its chemical behavior, and ultimately shapes its importance in both biological systems and industrial processes. Understanding atomic structure and the significance of the atomic number provides a foundation for comprehending the properties and behavior of all elements on the periodic table. The 11 protons in sodium are not just a number; they are the key to unlocking a world of chemical and physical interactions. From the formation of table salt to the intricate processes of life itself, the number 11, in the context of sodium's atomic structure, holds profound significance.
Latest Posts
Latest Posts
-
How Many Oxygen Atoms Are In Al2 So4 3
May 09, 2025
-
What Is The Percent Of 18
May 09, 2025
-
A Compound Contains Only Carbon Hydrogen And Oxygen
May 09, 2025
-
The Price Of An Item Was Lowered By 25
May 09, 2025
-
2 3 4 As A Improper Fraction
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are In Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.