How Many Orbitals In Each Sublevel
listenit
Apr 01, 2025 · 5 min read
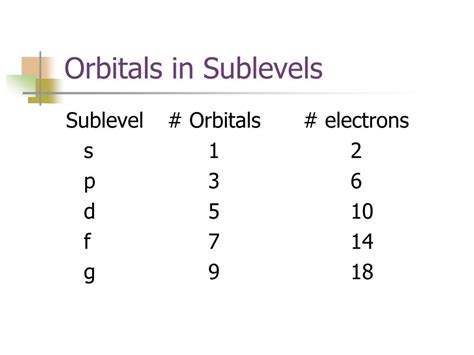
Table of Contents
How Many Orbitals in Each Sublevel? A Deep Dive into Atomic Structure
Understanding the arrangement of electrons within an atom is fundamental to chemistry. This arrangement, governed by quantum mechanics, dictates an atom's properties and how it interacts with other atoms. A key component of this understanding is grasping the concept of orbitals and sublevels within each electron shell. This article will delve into the specifics of how many orbitals exist within each sublevel (s, p, d, and f), explaining the underlying principles and their implications.
The Quantum Mechanical Model and Electron Configuration
Before diving into the number of orbitals, let's briefly review the quantum mechanical model of the atom. This model describes electrons not as particles orbiting the nucleus in fixed paths, but as existing in regions of space called orbitals. These orbitals are defined by a set of quantum numbers:
-
Principal Quantum Number (n): This number defines the electron shell and its energy level.
ncan be any positive integer (1, 2, 3, etc.). Largernvalues indicate higher energy levels and greater distance from the nucleus. -
Azimuthal Quantum Number (l): This number defines the sublevel within a shell.
lcan range from 0 ton-1. Each value oflcorresponds to a specific sublevel:l = 0: s sublevell = 1: p sublevell = 2: d sublevell = 3: f sublevel
-
Magnetic Quantum Number (ml): This number specifies the orbital within a sublevel.
mlcan range from -lto +l, including 0. This means each sublevel contains a specific number of orbitals. -
Spin Quantum Number (ms): This number describes the intrinsic angular momentum (spin) of the electron, which can be either +1/2 or -1/2. Each orbital can hold a maximum of two electrons, one with spin up (+1/2) and one with spin down (-1/2), according to the Pauli Exclusion Principle.
The Number of Orbitals in Each Sublevel
Now, let's address the core question: how many orbitals are in each sublevel? This is directly determined by the magnetic quantum number (ml).
s Sublevel (l = 0)
The s sublevel has only one possible value for ml: ml = 0. Therefore, the s sublevel contains only one orbital. This orbital is spherical in shape and is designated as the 1s, 2s, 3s, and so on, depending on the principal quantum number.
p Sublevel (l = 1)
For the p sublevel, l = 1. The possible values for ml are -1, 0, and +1. This means the p sublevel contains three orbitals. These orbitals have a dumbbell shape and are oriented along the x, y, and z axes, commonly designated as px, py, and pz.
d Sublevel (l = 2)
In the d sublevel, l = 2. The possible values for ml are -2, -1, 0, +1, and +2. Consequently, the d sublevel contains five orbitals. These orbitals have more complex shapes than s and p orbitals.
f Sublevel (l = 3)
For the f sublevel, l = 3. The values for ml range from -3 to +3, including 0. This gives a total of seven possible values, meaning the f sublevel contains seven orbitals. The shapes of f orbitals are even more intricate than d orbitals.
Visualizing Orbitals and Sublevels
Understanding the shapes of orbitals is crucial to visualizing the electron distribution within an atom. While precise depictions are complex, simplified representations often used in textbooks help illustrate the key differences:
- s orbitals: Spherical, with increasing size as the principal quantum number (n) increases.
- p orbitals: Dumbbell-shaped, oriented along the x, y, and z axes.
- d orbitals: More complex shapes, including cloverleaf and donut shapes.
- f orbitals: Even more complex shapes, with multiple lobes and nodal planes.
Electron Configuration and the Filling of Orbitals
The number of orbitals in each sublevel directly impacts the electron configuration of an atom. Electrons fill orbitals according to the Aufbau principle, which states that electrons occupy the lowest energy levels first. This filling is also governed by Hund's rule (electrons fill orbitals individually before pairing up) and the Pauli exclusion principle (each orbital can hold a maximum of two electrons with opposite spins).
For example, consider the element nitrogen (N), which has seven electrons. Its electron configuration is 1s²2s²2p³. This means:
- Two electrons fill the 1s orbital.
- Two electrons fill the 2s orbital.
- Three electrons fill the three 2p orbitals individually, according to Hund's rule.
Implications for Chemical Bonding and Reactivity
The arrangement of electrons in orbitals and sublevels directly influences an atom's chemical behavior. The outermost electrons, known as valence electrons, are particularly important because they participate in chemical bonding. The number of valence electrons, determined by the electron configuration, dictates an atom's bonding capacity and reactivity. For example, elements in the same group (vertical column) of the periodic table have the same number of valence electrons and often exhibit similar chemical properties.
Beyond the Basics: Hybrid Orbitals and Molecular Orbitals
The simple atomic orbital model described above forms the foundation for understanding more complex concepts in chemistry, such as:
-
Hybrid Orbitals: These are formed by the combination of atomic orbitals within the same atom. Hybrid orbitals explain the bonding geometries observed in many molecules.
-
Molecular Orbitals: These are formed by the combination of atomic orbitals from different atoms. Molecular orbitals describe the electron distribution in molecules and are crucial for understanding molecular properties like bonding strength and stability.
Conclusion: Orbitals, Sublevels, and the Foundation of Chemistry
Understanding the number of orbitals in each sublevel—one for s, three for p, five for d, and seven for f—is fundamental to grasping atomic structure and the behavior of atoms and molecules. This knowledge underpins our understanding of electron configuration, chemical bonding, and the periodic properties of elements. While the quantum mechanical model might seem abstract at first, its implications are far-reaching and essential for comprehending the world around us at the atomic level. This knowledge forms the bedrock of countless chemical phenomena and is indispensable for anyone pursuing studies in chemistry or related fields. Further exploration into advanced concepts like hybrid and molecular orbitals will reveal even deeper insights into the intricate workings of the atomic world.
Latest Posts
Latest Posts
-
33 As A Fraction In Simplest Form
Apr 02, 2025
-
How Many Chromosomes Does Each Daughter Cell Have
Apr 02, 2025
-
Does A Period Go Before Or After Quotations
Apr 02, 2025
-
How To Go From Vertex Form To Factored Form
Apr 02, 2025
-
Who Said I Am The State
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Orbitals In Each Sublevel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
